No products in the cart.
Boric acid Reneval, 3% solution 25 ml
€6.25 €5.56
Description
Pharmacotherapeutic group: antiseptic.
ATX code: D08AD
Pharmacological properties
Pharmacodynamics
Antiseptic agent; coagulates proteins (including enzymes) of the microbial cell, breaks the permeability of the cell wall.
Pharmacokinetics
Well penetrates through skin and mucous membranes; slowly excreted and can accumulate in organs and tissues. It is excreted by the kidneys – 50% (within 12 hours), the remaining amount – within 5-7 days.
Indications
Indications
Otitis externa (acute and chronic) without damage to the eardrum.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antiseptic.
ATX code: D08AD
Pharmacological properties
Pharmacodynamics
Antiseptic; coagulates proteins (including enzymes) of the microbial cell, disrupts the permeability of the cell wall.
Pharmacokinetics
Penetrates well through the skin and mucous membranes; is slowly excreted and can accumulate in organs and tissues. Excreted by the kidneys – 50% (within 12 hours), the rest – within 5-7 days.
Special instructions
Special instructions
Avoid contact with mucous membranes.
The drug is used in patients over 18 years of age.
Effect of the drug on the ability to drive vehicles and machinery
The drug does not affect the ability to drive or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
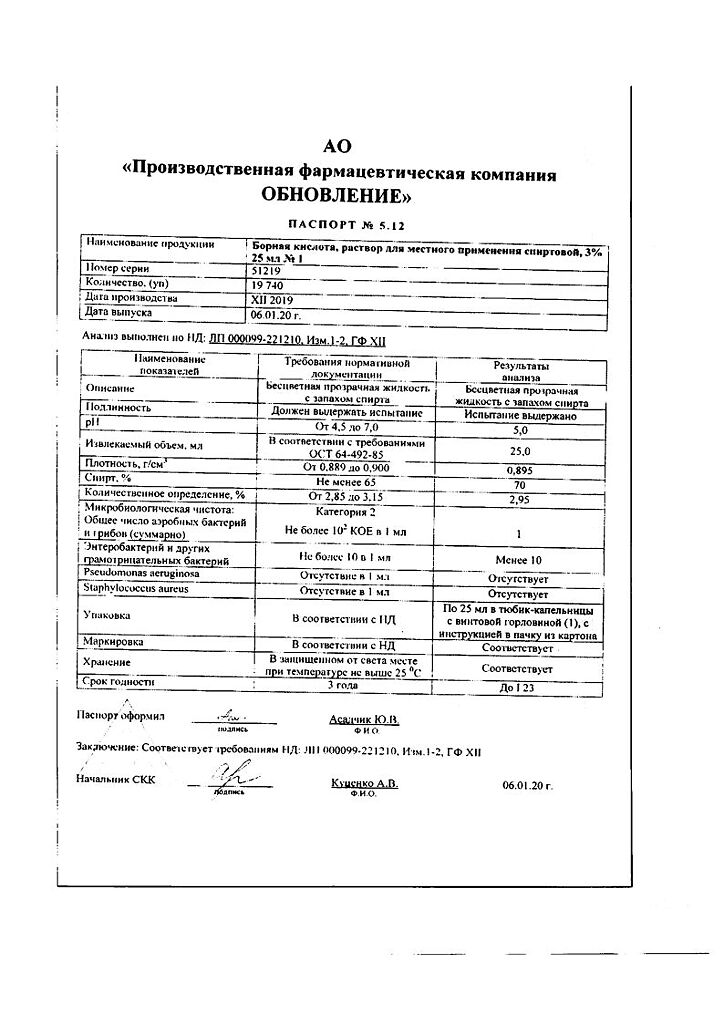
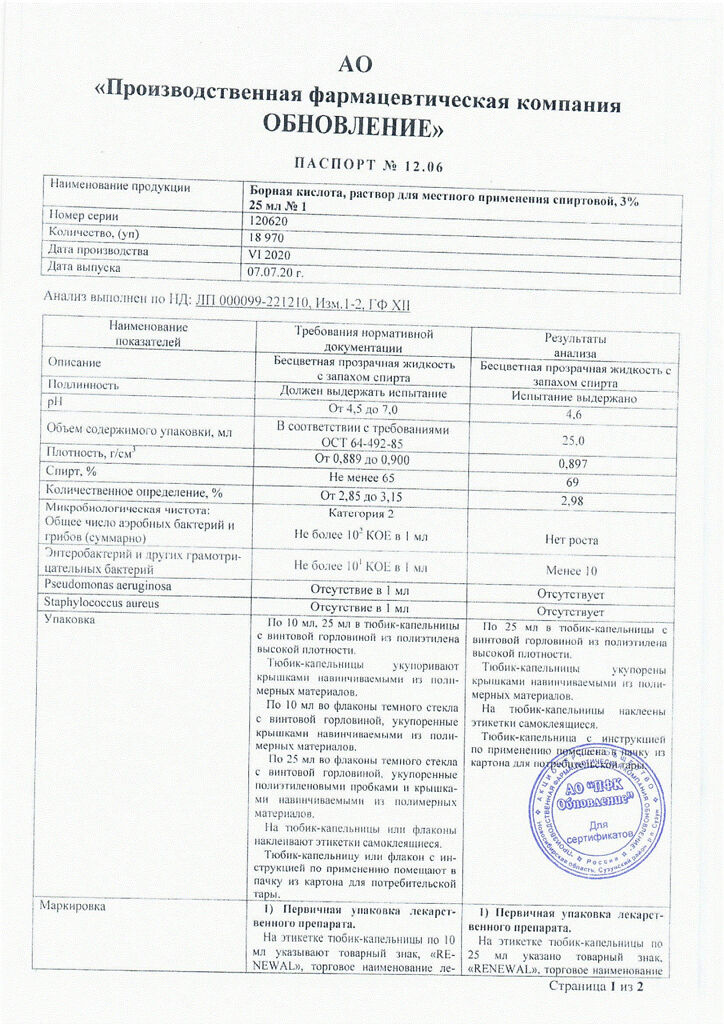
Boric acid
Composition
Composition
Composition per 1000 ml
Active ingredient:
Boric acid – 30 g
Excipient:
ethanol (ethyl alcohol) 70% – up to 1000 ml
Contraindications
Contraindications
Hypersensitivity, chronic renal failure, perforation of the eardrum, pregnancy, children under 18 years of age, breastfeeding.
Side Effects
Side Effects
Local reactions: itching, burning, hyperemia of the skin of the external auditory canal. Allergic reactions.
If any of the side effects indicated in the instructions worsen, or any other side effects not specified in the instructions are noted, you should immediately inform your doctor.
Interaction
Interaction
Not studied.
Overdose
Overdose
Symptoms of acute intoxication (with accidental ingestion): nausea, vomiting, diarrhea, gastralgia, dysfunction of the cardiovascular system, stimulation or depression of the central nervous system, hyperpyrexia, erythematous rashes followed by desquamation (possible death within 5-7 days), impaired renal and liver function (including jaundice), circulatory collapse, shock, incl. with fatal outcome.
Treatment: symptomatic. Blood transfusion, hemo- and peritoneal dialysis.
Storage conditions
Storage conditions
In a pack at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Update of PFC JSC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | In a package at a temperature not exceeding 25 °С. Keep out of reach of children. |
| Manufacturer | Update PFC AO, Russia |
| Medication form | topical solution |
| Brand | Update PFC AO |
Other forms…
Related products
Buy Boric acid Reneval, 3% solution 25 ml with delivery to USA, UK, Europe and over 120 other countries.