No products in the cart.
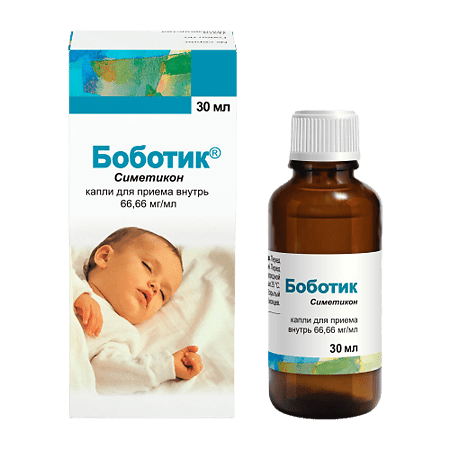
Bobotik, drops 66.66mg/ml 30 ml
€10.85 €9.49
Description
BOBOTIC has a foam-forming, antipyrogonic action.
Pharmacodynamics
Simethicone (activated dimethicone) is a combination of methylated linear siloxane polymers stabilized with trimethylsiloxyl groups with silicon dioxide. By reducing the surface tension at the interface, it hinders the formation and promotes the destruction of gas bubbles in the intestinal contents and mucus of the gastrointestinal tract (GIT).
The gases released during this process can be absorbed by the intestinal wall or eliminated through peristalsis. This prevents the formation of large gas-mucous conglomerates that cause painful abdominal bloating. In sono- and radiography it prevents image defects; it promotes better irrigation of mucous coat of large intestine with contrast preparations, preventing rupture of contrast film.
Due to chemical inertness it does not affect the microorganisms and enzymes present in the gastrointestinal tract. It does not reduce absorption of food, does not change the reaction and volume of gastric juice.
Pharmacokinetics
Simethicone after oral administration is not absorbed from the GI tract and is excreted unchanged through the intestine.
Indications
Indications
– Increased gas formation and accumulation of gases in the gastrointestinal tract (colic, feeling of fullness in the abdominal cavity, flatulence, including in the postoperative period, Remgeld syndrome, aerophagia);
– preparation for diagnostic studies of the abdominal and pelvic organs (radiography, sonography, gastroscopy and duodenoscopy – prevention of foam formation).
Pharmacological effect
Pharmacological effect
BOBOTIC has an antifoaming, carminative effect.
Pharmacodynamics
Simethicone (activated dimethicone) is a combination of methylated linear siloxane polymers stabilized by trimethylsiloxy groups with silica. By reducing the surface tension at the interface, it impedes the formation and promotes the destruction of gas bubbles in the intestinal contents and mucus of the gastrointestinal tract (GIT).
The gases released during this process can be absorbed by the intestinal walls or excreted through peristalsis. This prevents the formation of large gas-mucus conglomerates that cause painful bloating. Prevents the occurrence of image defects during sonography and radiography; promotes better irrigation of the colon mucosa with contrast agents, preventing rupture of the contrast film.
Due to chemical inertness, it does not affect microorganisms and enzymes present in the gastrointestinal tract. Does not reduce food absorption, does not change the reaction and volume of gastric juice.
Pharmacokinetics
Simethicone after oral administration is not absorbed from the gastrointestinal tract and is excreted unchanged through the intestines.
Active ingredient
Active ingredient
Simethicone
Composition
Composition
1 ml of drops for oral administration contains:
Active ingredients:
simethicone emulsion 30% 222.2 mg,
simethicone 66.66 mg;
Excipients:
sodium saccharinate,
methyl parahydroxybenzoate,
propyl parahydroxybenzoate,
carmellose sodium,
citric acid monohydrate,
raspberry flavoring,
purified water.
Pregnancy
Pregnancy
During pregnancy, the use of the drug is possible only in cases where the expected benefit to the mother outweighs the potential risk to the fetus.
There are no data on the safety of the drug in nursing women. During breastfeeding, the drug should be used only if absolutely necessary.
Contraindications
Contraindications
– Hypersensitivity to simethicone and/or other components of the drug Bobotik;
– intestinal obstruction;
– obstructive gastrointestinal diseases;
– newborns up to 28 days of life.
Side Effects
Side Effects
Allergic reactions may develop.
Interaction
Interaction
Simethicone may cause malabsorption of oral anticoagulants.
Overdose
Overdose
There are no reports of overdose.
Simethicone is not absorbed from the gastrointestinal tract and an overdose does not pose a threat to life and health.
Storage conditions
Storage conditions
In a place protected from light, at a temperature of 15–25 °C.
Shelf life
Shelf life
3 years. After opening – 2 months.
Do not use after the expiration date indicated on the package.
Manufacturer
Manufacturer
Polpharma JSC, Poland
Additional information
| Shelf life | 3 years. After opening – 2 months. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | In a light-protected place at 15-25 °C. |
| Manufacturer | Polpharma S.A., Poland |
| Medication form | oral drops |
| Brand | Polpharma S.A. |
Related products
Buy Bobotik, drops 66.66mg/ml 30 ml with delivery to USA, UK, Europe and over 120 other countries.