No products in the cart.
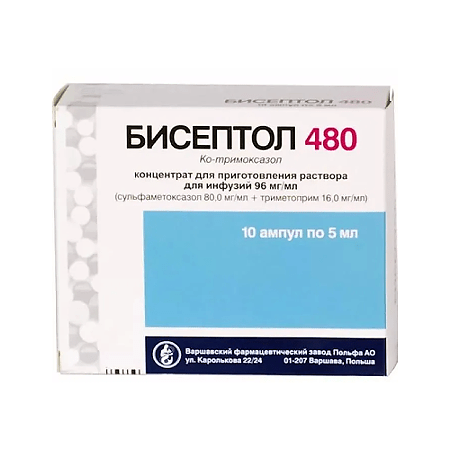
Biseptol 480, concentrate 80+16 mg/ml 5 ml 10 pcs.
€12.28 €10.23
Out of stock
(E-mail when Stock is available)
Description
A combination antibacterial drug containing sulfamethoxazole with medium duration of action inhibiting folic acid synthesis by competitive antagonism with para-aminobenzoic acid and trimetholrim inhibitor of bacterial dihydrofolic acid reductase. The combination of both drugs gives an energic effect of antibacterial action due to which bacterial resistance is less frequent compared to other drugs.
Biseptol has a wide spectrum of antibacterial action. It is active against : Streptococcus (Streptococcus pneumoniae), Neisseria meningitidis, Neisseria gonorrhoeae (including enterotoxigenic strains), Staphylococcus, Escherichia coli, Klebsiella, Enterobacter, Proteus mirabilis, Proteus arr, Haemophilus influenzae, Salmonella spp. (including Salmonella typhi and Salmonella paratyphi), Vibrio cholerae, Bacillus anthracis, Listeria spp., Nocardia asteroides, Bordclella pertussis, Enterococcus faecalis, Pasteurella spp, Brucella spp., Mycobacterium spp. (including Mycobacterium leprae), Citrobacter, Enterobacter spp., Legionella pneumonia, Providencia, some Pseudomonas species (except P. aerugenosa), Serratia marcescens, Yersinia spp, Morganella spp., Chlamydia spp. (including Chlamydia trachomatis, Chlamydia psittaci), Shigella, Plasmodium spp., Toxoplasma gondii, Pneumocystis carini, Actinomyces israelii, Coccidioides immitis, Histoplasma capsulatum, Leishmania spp.
Resistant to the drug: Corynebacterium spp., Pseudomonas aerugenosa, Mycobacterium tuberculosis, Troponema spp., Leptospira spp., viruses.
Inhibits the activity of E. coli, leads to a decrease in the synthesis of thiamine, riboflavin, nicotinic acid and other B vitamins in the intestine. The duration of the therapeutic effect is 7 hours.
Indications
Indications
acute and chronic infections of the genitourinary organs: urethritis, pyelonephritis, cystitis, pyelitis, prostatitis, epididymitis, gonorrhea, chancroid, lymphogranuloma venereum, inguinal granuloma;
respiratory tract infections: bronchitis (acute and chronic), bronchiectasis, lobar pneumonia, bronchopneumonia, Pneumocystis pneumonia, pleural empyema, lung abscess;
infections of the ENT organs: otitis media, sinusitis, laryngitis, tonsillitis, scarlet fever;
gastrointestinal infections: typhoid fever, paratyphoid fever, salmonella carriage, cholera, dysentery, cholecystitis, cholangitis, gastroenteritis caused by enterotoxic strains of E. coli;
infections of the skin and soft tissues: acne, furunculosis, pyoderma, abscess and wound infections, infections after surgical interventions;
sepsis, acute brucellosis, toxoplasmosis, osteomyelitis, osteoarticular infections, South American blastomycosis, malaria (Plasmodium falciparum), whooping cough (as part of complex therapy).
Pharmacological effect
Pharmacological effect
A combined antibacterial drug containing sulfamethoxazole, which has an average duration of action, inhibits the synthesis of folnic acid by competitive antagonism with para-aminobenzoic acid, as well as trimetolrim, an inhibitor of bacterial dihydrofolic acid reductase. The combination of both drugs gives an energetic effect of antibacterial action, and therefore bacterial resistance appears less frequently compared to other drugs.
Biseptol has a wide spectrum of antibacterial action. It is active against: Streptococcus (Streptococcus pneumoniae), Neisseria meningitidis, Neisseria gonorrhoeae (including enterotoxogenic strains), Staphylococcus, Escherichia coli, Klebsiella, Enterobacter, Proteus mirabilis, Proteus spp., Haemophilus influenzae, Salmonella spp. (including Salmonella typhi and Salmonella paratyphi), Vibrio cholerae, Bacillus anthracis, Listeria spp., Nocardia asteroides, Bordclella pertussis, Enterococcus faecalis, Pasteurella spp., Brucella spp., Mycobacterium spp. (including Mycobacterium leprae), Citrobacter, Enterobacter spp., Legionella pneumonia, Providencia, some species of Pseudomonas (except P.аerugenosa), Serratia marcescens, Yersinia spp., Morganella spp., Chlamydia spp. (including Chlamydia trachomatis, Chlamydia psittaci), Shigella, Plasmodium spp., Toxoplasma gondii, Pneumocystis carini, Actinomyces israelii, Coccidioides immitis, Histoplasma capsulatum, Leishmania spp.
Resistant to the drug: Corynebacterium spp., Pseudomonas aerugenosa, Mycobacterium tuberculosis, Troponema spp., Leptospira spp., viruses.
Inhibits the vital activity of E. coli, leading to a decrease in the synthesis of thiamine, riboflavin, nicotinic acid and other B vitamins in the intestines. The duration of the therapeutic effect is 7 hours.
Special instructions
Special instructions
In AIDS patients treated with cotrimoxazole due to Pneumocystis carinii infection, undesirable effects are more often observed: skin rashes, increased body temperature, leukopenia.
It is advisable to determine the plasma concentration of sulfamethoxazole every 2-3 days immediately before the next infusion. If the concentration of sulfamethoxazole exceeds 150 mcg/ml, treatment should be interrupted until it decreases below 120 mcg/ml.
During long-term treatment, studies of peripheral blood, the functional state of the liver and kidneys should be systematically carried out.
For elderly patients, additional administration of folic acid (3-6 mg/day) is recommended, which does not significantly interfere with the antimicrobial activity of the drug. Particular caution should be exercised when treating elderly patients with suspected underlying folate deficiency.
To prevent crystalluria, it is recommended to maintain a sufficient volume of urine excreted.
The likelihood of toxic and allergic complications of sulfonamides increases significantly with complications of the filtration function of the kidneys.
During treatment, it is also not advisable to consume foods containing large quantities of PABA-green parts of plants (cauliflower, spinach, legumes), carrots, tomatoes.
Excessive exposure to sunlight and ultraviolet radiation should be avoided.
It is not recommended for use for tonsillitis, pharyngitis caused by group A beta-hemolytic streptococcus due to widespread strain resistance.
Impact on the ability to drive vehicles and operate machinery
The drug does not affect the ability to drive vehicles or maintain moving machinery.
Active ingredient
Active ingredient
Co-trimoxazole [Sulfamethoxazole, Trimethoprim]
Composition
Composition
Active substances:
sulfamethoxazole 80.00 mg + trimethoprim 16.00 mg
Excipients:
propylene glycol,
sodium hydroxide,
ethanol,
benzyl alcohol,
sodium metabisulfite,
water for d/i.
Contraindications
Contraindications
megaloblastic anemia due to folic acid deficiency, aplastic anemia, B12-deficiency anemia, agranulocytosis, leukopenia;
deficiency of glucose-6-phosphate dehydrogenase;
hyperbilirubinemia in children;
hepatic and/or renal failure; creatinine clearance less than 15 ml/min);
age up to 6 years (only for intramuscular administration);
pregnancy;
lactation period;
increased individual sensitivity to sulfonamides or trimethoprim.
The drug should not be used in premature infants, newborns and infants under 2 months of age.
Use with caution for folic acid deficiency, bronchial asthma, thyroid diseases, liver and kidney dysfunction.
Side Effects
Side Effects
Biseptol is usually well tolerated by patients. However, the following effects may be observed:
From the gastrointestinal tract: anorexia, gastritis, abdominal pain, glossitis, stomatitis, cholestasis, increased activity of liver transaminases, hepatitis, pseudomembranous enterocolitis, nausea, vomiting, diarrhea, liver necrosis.
From the central nervous system: headaches and dizziness. In some cases – aseptic meningitis, depression, apathy, tremor, peripheral neuritis.
From the respiratory system: bronchospasm, pulmonary infiltrates.
From the hematopoietic organs: rarely – neutropenia, agranulocytosis, megaloblastic anemia, leukopenia, thrombocytopenia, hypoprothrombinemia.
From the urinary system: polyuria, interstitial nephritis, impaired renal function, crystalluria, hematuria, increased urea content, hypocreatininemia, toxic nephropathy with oliguria and anuria.
From the musculoskeletal system: arthralgia, myalgia.
Allergic reactions: skin rashes and itching, photosensitivity, rash, erythema multiforme, exfoliative dermatitis, allergic myocarditis, fever, Quincke’s edema, redness of the sclera.
Local reactions: thrombophlebitis (at the site of venipuncture), pain at the injection site.
Other: hypoglycemia
Interaction
Interaction
Biseptol enhances the effect of phenytoin, oral hypoglycemic agents, warfarin derivatives (extension of prothrombin time, bleeding).
In elderly patients, in combination with diuretics (in particular, thiazide diuretics), the risk of developing thrombocytopenia increases.
Simultaneous use with cyclosporine reduces its concentration in the blood.
The drug should not be administered intravenously in combination with medications and solutions containing bicarbonates.
Biseptol is pharmaceutically compatible with the following drugs: dextrose for IV infusion 5%, sodium chloride for IV infusion 0.9%, a mixture of 0.18% sodium chloride and 4% dextrose for IV infusion, 6% dextran 70 for IV infusion in 5% dextrose or saline, 10% dextran 40 for IV infusion in 5% dextrose or saline, Ringer’s solution for injection.
Increases the anticoagulant activity of indirect coagulants, enhances the effect of hypoglycemic agents and methotrexate.
Reduces the intensity of hepatic metabolism of phenytoin (extends its T1/2 by 39%) and warfarin, enhancing their effect.
Rifampicin reduces T1/2 of trimethoprim.
Pyrimethamine in doses exceeding 25 mg/week increases the risk of developing megaloblastic anemia.
Diuretics (usually thiazides) increase the risk of thrombocytopenia.
The effect is reduced by benzocaine, procaine, procainamide and other drugs, the hydrolysis of which results in the formation of PABA.
Between diuretics (thiazides, furosemide, etc.) and oral hypoglycemic drugs (sulfonylurea derivatives) on the one hand and antimicrobial sulfonamides on the other, the development of a cross-allergic reaction is possible.
Pheninoin, barbiturates, PAS increase the manifestations of folic acid deficiency.
Salicylic acid derivatives enhance the effect.
Ascorbic acid, hexamethylenetetramine and other drugs that acidify urine increase the risk of developing crystalluria.
Colesteramine reduces absorption, so it should be taken 1 hour after or 4-6 hours before taking cotrimoxazole.
Reduces the reliability of oral contraception (inhibits intestinal microflora and reduces the enterohepatic circulation of hormonal compounds).
Overdose
Overdose
Symptoms: nausea, vomiting, intestinal colic, dizziness, headache, drowsiness, depression, fainting, confusion, blurred vision, fever, hematuria, crystalluria; with prolonged overdose – thrombocytopenia, leukopenia, megaloblastic anemia, jaundice.
Treatment: gastric lavage, acidification of urine increases the excretion of trimethoprim, fluid intake orally, intramuscularly – 5-15 mg / day, calcium folinate (eliminates the effect of trimethoprim on the bone marrow), in case of inhibition of the hematopoietic functions of the bone marrow caused by trimethoprim, folic acid preparations are used intramuscularly to stimulate erythropoiesis (3-6 mg / day. Course of treatment – 5-7 days), if necessary – hemodialysis.
Functional features
Functional features
The drug quickly penetrates the tissues and biological fluids of the body.
Well distributed. Penetrates through the blood-brain barrier, the placental barrier and into breast milk. In the lungs and urine it creates concentrations exceeding the content in plasma. To a lesser extent, they accumulate in bronchial secretions, vaginal secretions, secretions and tissues of the prostate gland, middle ear fluid, cerebrospinal fluid, bile, bones, saliva, aqueous humor of the eye, breast milk, interstitial fluid. The distribution of both drugs is different: sulfamethoxazole is distributed exclusively in the extracellular space, and trimethoprim is distributed both intracellularly and in the extracellular space. Plasma protein binding is 66% for sulfamethoxazole and 45% for trimethoprim. Both drugs are metabolized in the liver.
Sulfamethoxazole is metabolized to a greater extent (with the formation of acetylated derivatives); the metabolites do not have antimicrobial activity.
Excreted by the kidneys, both by filtration and by active secretion by the tubules, in the form of metabolites (80% within 72 hours) and unchanged (20% sulfamethoxazole, 50% trimethoprim), the concentration of active substances in the urine is much higher than in the blood. A small amount of the drug is excreted through the intestines. T1/2 of sulfamethoxazole – 9-11 hours, trimethoprim – 10-12 hours, in children – significantly less and depends on age: up to the first year – 7-8 hours, 1-10 years – 5-6 hours. In the elderly and patients with impaired renal function, T1/2 increases.
Storage conditions
Storage conditions
List B. In a place protected from light, at a temperature not exceeding 30°C.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Polpharma JSC, Poland
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | List B. In a place protected from light, at a temperature not exceeding 30°C. |
| Manufacturer | Polfa Warsaw Pharmaceutical Plant, Poland |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Polfa Warsaw Pharmaceutical Plant |
Other forms…
Related products
Buy Biseptol 480, concentrate 80+16 mg/ml 5 ml 10 pcs. with delivery to USA, UK, Europe and over 120 other countries.