No products in the cart.
Betaserk, tablets 16 mg 30 pcs
€17.70 €15.34
Description
Betaserk is a microcirculatory enhancer.
Pharmacodynamics
Betaserk (betahistine) acts mainly on histamine H1 and H3 receptors of the inner ear and vestibular nuclei of the CNS. By direct agonist effect on H1-receptors of the inner ear vessels and indirectly through effect on H3-receptors it improves micro-circulation and capillary permeability and normalizes the endolymph pressure in the labyrinth and cochlea.
At the same time, betahistine increases blood flow in the basilar arteries.
Betaserk® also has a pronounced central effect due to its effect on the H3-receptors of the vestibular nerve nuclei. It normalizes conduction in neurons of vestibular nuclei at brainstem level.
The clinical manifestation of these properties is reduction of the frequency and intensity of dizziness, decrease of tinnitus, improvement of hearing if it is reduced.
Indications
Indications
Meniere’s syndrome, characterized by the following main symptoms: dizziness (accompanied by nausea/vomiting); hearing loss (hearing loss); tinnitus.
symptomatic treatment of vestibular dizziness (vertigo).
Pharmacological effect
Pharmacological effect
Betaserc – improves microcirculation.
Pharmacodynamics
Betaserc (betahistine) acts mainly on histamine H1 and H3 receptors in the inner ear and vestibular nuclei of the central nervous system. Through a direct agonistic effect on H1 receptors of the vessels of the inner ear, as well as indirectly through an effect on H3 receptors, it improves microcirculation and capillary permeability, normalizes endolymph pressure in the labyrinth and cochlea.
At the same time, betahistine increases blood flow in the basilar arteries.
Betaserc® also has a pronounced central effect due to its influence on the H3 receptors of the vestibular nerve nuclei. Normalizes conductivity in the neurons of the vestibular nuclei at the level of the brain stem.
The clinical manifestation of these properties is a decrease in the frequency and intensity of dizziness, a decrease in tinnitus, and an improvement in hearing if it is reduced.
Special instructions
Special instructions
It should be used with caution in patients with a history of gastric or duodenal ulcers, in the second and third trimesters of pregnancy, as well as in children.
It must be taken into account that the desired clinical effect is achieved after several months of treatment.
For dyspeptic symptoms, betahistine is recommended to be taken during or after meals.
Active ingredient
Active ingredient
Betahistine
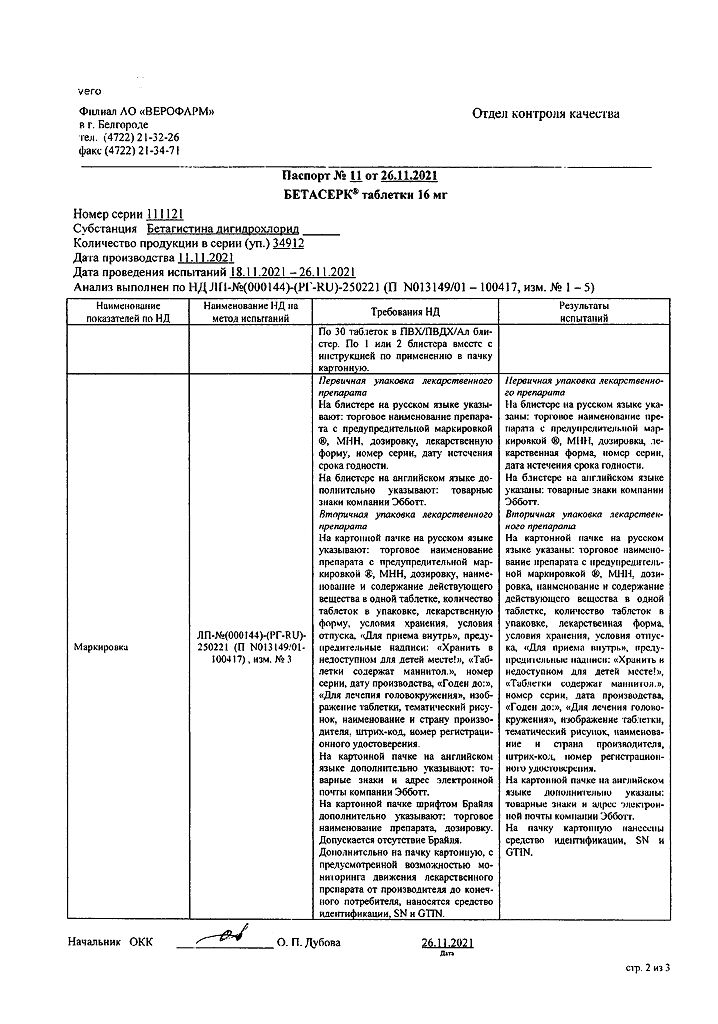
Composition
Composition
1 tablet contains:
Active substance:
betahistine dihydrochloride – 16 mg.
Excipients:
microcrystalline cellulose – 161.5 mg,
mannitol (E421) – 50 mg,
citric acid monohydrate – 5 mg,
colloidal silicon dioxide – 5 mg,
talc – 12.5 mg.
Pregnancy
Pregnancy
Pregnancy.
There is insufficient data available on the use of betahistine in pregnant women. Animal studies have shown no direct or indirect reproductive toxicity. Betahistine should not be used during pregnancy unless clearly needed.
Breast-feeding.
It is unknown whether betahistine is excreted in human breast milk. Betahistine is excreted in breast milk in rats. Animal studies have been limited to very high doses of the drug. The question of prescribing a drug to the mother should be made only after comparing the benefits of breastfeeding with the potential risk to the infant.
Fertility.
Animal studies (rats) have not shown any effect on fertility.
Contraindications
Contraindications
pheochromocytoma;
hypersensitivity to any of the components of the drug.
Betaserc® is not recommended for use in children under 18 years of age due to insufficient data on efficacy and safety.
Side Effects
Side Effects
Common: nausea and dyspepsia; headache;
Not known: hypersensitivity reactions, including anaphylactic reaction, vomiting, gastrointestinal pain, bloating, angioedema, urticaria, itching, rash.
Interaction
Interaction
In vivo studies aimed at studying interactions with other drugs have not been conducted.
Based on in vitro data, it can be assumed that there is no inhibition of the activity of isoenzymes of the cytochrome P450 system in vivo.
In vitro data have shown inhibition of betahistine metabolism by drugs that inhibit MAO, including MAO subtype B (eg, selegiline). Caution should be exercised when prescribing betahistine and MAO inhibitors (including MAO-B) simultaneously.
Betahistine is a histamine analogue, and the interaction of betahistine with histamine H1 receptor blockers could theoretically affect the effectiveness of one of these drugs.
The patient should tell the doctor if they are currently or recently taking any medications.
Overdose
Overdose
There are several known cases of overdose of Betaserc.
Symptoms: mild to moderate nausea, drowsiness, abdominal pain were observed in some patients after taking the drug in doses up to 640 mg.
More serious complications (convulsions, cardiopulmonary complications) have been observed with deliberate use of betahistine in increased doses, especially in combination with overdose of other drugs.
Treatment: symptomatic therapy.
Storage conditions
Storage conditions
In a dry place, at a temperature not exceeding 25 °C
Shelf life
Shelf life
1 year
Manufacturer
Manufacturer
Veropharm LLC, Russia
Additional information
| Shelf life | 1 year |
|---|---|
| Conditions of storage | In a dry place, at a temperature not exceeding 25 °C |
| Manufacturer | Veropharm AO, Russia |
| Medication form | pills |
| Brand | Veropharm AO |
Other forms…
Related products
Buy Betaserk, tablets 16 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.