No products in the cart.
Balm Gold Star, ointment 4 g
€5.07 €4.51
Description
- Hypnea.
- Sniffing.
- In case of insect bites.
- Headache.
- Colds.
Indications
Indications
Showing results upon request As a symptomatic remedy in the complex treatment of acute respiratory diseases (ARI), influenza, headaches, dizziness, rhinitis (runny nose), insect bites.
Hypersensitivity to the components of the drug.
Damage to the skin, the presence of skin diseases in the areas where the drug is intended to be used (pyoderma, dermatitis, eczema).
Epilepsy, tendency to seizures, spasmophilia, bronchial asthma, whooping cough. Due to the lack of special studies, the use of the drug during pregnancy and breastfeeding is contraindicated. If it is necessary to use the drug during lactation, breastfeeding should be stopped for the period of use of the drug. Directions for use and dosage: rub the ointment into the skin of the wings of the nose and temporal area. For insect bites, apply the drug to the bite site.
Balm Golden Star is intended for external use only. If there is no improvement after treatment or new symptoms appear, you should consult your doctor. with your doctor before using the drug.
Hypersensitivity reactions are possible, when the first signs of which appear (individual elements of rash, sore throat, itching, redness), the use of the drug should be stopped and consult a doctor. If side effects occur, including those not described in this instruction, you should stop using the drug and inform your doctor and/or the organization responsible for pharmacovigilance, whose contacts are given at the end of the instructions. In order to obtain new information on the safety of the drug, medical professionals are recommended to report each case of an adverse reaction that occurs during the use of Golden Star Balm. This allows you to constantly monitor the benefit/risk ratio of the drug.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Local irritant of natural origin.
ATX code: R05X
Pharmacological properties
Pharmacodynamics
Balm Golden Star has a locally irritating, distracting, antiseptic, weak local anti-inflammatory effect. The effect of the drug is due to the content of terpene derivatives (levomenthol and camphor) and essential oils in its composition. Levomenthol is a local irritant and has an antimicrobial, anti-inflammatory effect on the skin in terms of pain and itching.
The local effect is accompanied by vasoconstriction, a feeling of cold, turning into a feeling of lightness. Camphor has an analgesic, anti-inflammatory leaf oil, local anesthetic, and antiseptic effect. Pharmacokinetics The drug has a local effect, its components are practically not absorbed through the skin and do not have a systemic effect.
Active ingredient
Active ingredient
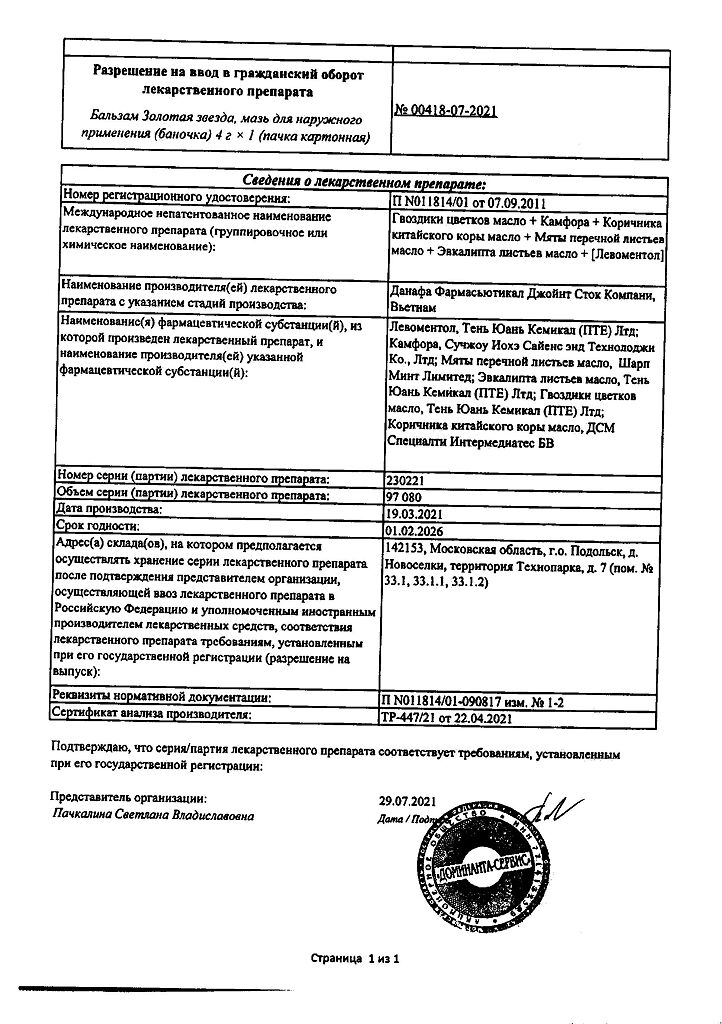
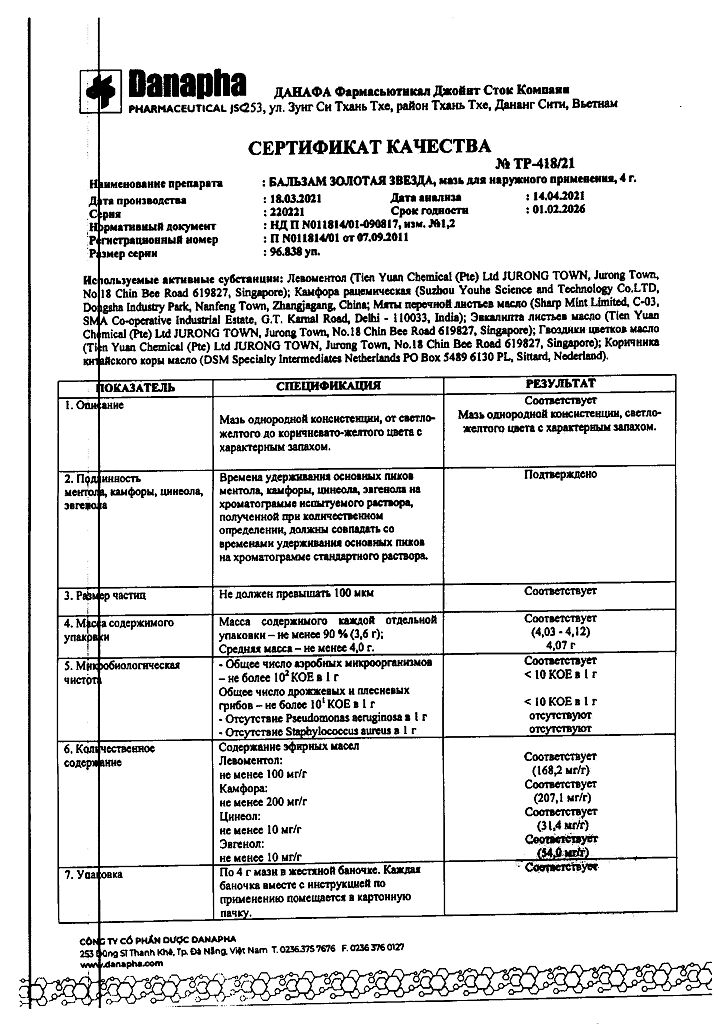
Clove oil, Camphor, Cinnamon oil, Peppermint oil, Peppermint oil, Thymol, Chloral hydrate, Eucalyptus oil
Composition
Composition
Levomenthol 0.455 g,
Racemic camphor 0.910 g,
Peppermint oil 0.590 ml,
Eucalyptus leaf oil 0.140 ml,
Carnation flowers oil 0.228 ml,
Chinese cinnamon oil 0.053 ml.
And also excipients: Beeswax – 0.360 g, paraffin – 0.750 g, anhydrous lanolin – 0.460 g, petroleum jelly – 0.230 g, liquid paraffin – 0.100 ml.
Pregnancy
Pregnancy
Due to the lack of special studies, the use of the drug during pregnancy and breastfeeding is contraindicated. If it is necessary to use the drug during the period, feeding should be stopped for the period of use of the drug.
Contraindications
Contraindications
Hypersensitivity to the components of the drug.
Children under 2 years of age.
Damage to the skin.
The presence of skin diseases in the areas where the drug is intended to be used.
Side Effects
Side Effects
Classified by frequency and system-organ class. The MedDRA frequency is determined as follows:
very common (1/10), common (1/100 to 1/10), uncommon (1/1,000 to 1/100), rare (>1/10,000 to 1/1,000), very rare (1/10,000), frequency unknown (cannot be estimated from the available data).
Immune system disorders, including hypersensitivity
Uncommon: skin itching, urticaria, rash.
Rarely – angioedema.
Frequency unknown – Stevens-Johnson syndrome.
Visual disorders
Often – lacrimation.
Disorders of the skin and subcutaneous tissues
Often – redness of the skin and irritation of the skin in the area where the drug is applied.
Rarely – allergic dermatitis.
Hypersensitivity reactions are possible, and when the first signs appear (individual elements of rash, sore throat, itching, redness), use of the drug should be stopped and consult a doctor.
If side effects occur, including those not described in this instruction, you should stop using the drug and inform your doctor and/or the organization responsible for pharmacovigilance, whose contacts are given at the end of the instructions. In order to obtain new information on the safety of the drug, medical professionals are recommended to report each case of an adverse reaction that occurs during the use of Golden Star Balm. This allows you to constantly monitor the benefit/risk ratio of the drug.
To date, there have been no cases of overdose. In case of accidental ingestion of the drug, symptoms of overdose include gastrointestinal (nausea, vomiting, diarrhea, abdominal pain) neurological disorders (headache, dizziness, hot flashes/flushes, convulsions, respiratory depression and coma). Treatment is symptomatic. Do not induce vomiting due to the risk
Interaction
Interaction
Not described. The drug is not recommended for use simultaneously with other drugs for external use in areas where Golden Star Balm is applied due to the lack of data on interaction.
Recommendations for use
Recommendations for use
Externally.
For headaches and dizziness, Golden Star Balm is rubbed into the skin of the temporal and occipital areas.
For rhinitis (runny nose), acute respiratory infections – the ointment is rubbed into the skin of the wings of the nose and the temporal region.
For insect bites, apply the drug to the bite site. Balm Golden Star is intended for external use only. If there is no improvement after treatment or new symptoms appear, you should consult your doctor. Use the drug only according to the method of use and in the doses indicated in the instructions. If necessary, consult
Storage conditions
Storage conditions
Store at 30°C. Keep out of the reach of children!
Shelf life
Shelf life
5 years. Do not use after expiration date.
Manufacturer
Manufacturer
Danafa Pharmaceutical Joint, Vietnam
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | In a dry, light-protected place at 12-15 °C. |
| Manufacturer | Danafa Pharmaceutical Joint, Vietnam |
| Medication form | topical ointment |
| Brand | Danafa Pharmaceutical Joint |
Other forms…
Related products
Buy Balm Gold Star, ointment 4 g with delivery to USA, UK, Europe and over 120 other countries.