No products in the cart.
Azafen, tablets 25 mg 50 pcs
€5.64 €4.70
Out of stock
(E-mail when Stock is available)
Description
Tricyclic antidepressant. By blocking reverse neuronal uptake of monoamines by presynaptic membranes, it increases their content in the synaptic cleft, which leads to relief of depression symptoms. The tholeptic action of the drug is combined with sedative activity and anxiolytic effect. Unlike tricyclic antidepressants it does not have choline-blocking properties, does not affect the activity of MAOIs, does not have cardiotoxic effect.
Pharmacokinetics
Intake
It is quickly and completely absorbed from the gastrointestinal tract. Bioavailability is about 80%. Tmax after taking the tablets is 2 hours.
The active substance in tablets Azafen MB is contained in a special carrier matrix allowing gradual release of pipofesine in the gastrointestinal tract. Released pipofesine is quickly and almost completely absorbed from the gastrointestinal tract. When single oral administration of Azafen MB 150 mg tablet Cmax in blood is reached after 3-4 hours and is 111 ng/ml.
Distribution and metabolism
The binding to plasma proteins is 90%.
It is largely biotransformed in the liver with the formation of inactive metabolites.
In in vitro study it was shown that pipofezine is not a substrate of CYP2C9, CYP2C19, CYP2D6 and CYP3A4 isoenzymes, but is predominantly metabolized under the influence of CYP1A2.
The T1/2 after taking the tablets is 16 h, the T1/2 after taking the tablets with modified release is 9 h. It is excreted mainly by the kidneys.
The drug retention time in the body (MRT) is on the average 13.4 hours (from 10 to 20 hours). When repeated administration of the drug in plasma concentration fluctuations between two doses are smoothed out. It is eliminated from the body mainly by the kidneys.
Indications
Indications
Depressive episodes of mild to moderate severity (including in chronic somatic diseases).
Pharmacological effect
Pharmacological effect
Tricyclic antidepressant. By blocking the reverse neuronal uptake of monoamines by presynaptic membranes, it increases their content in the synaptic cleft, which leads to relief of symptoms of depression. The thymoleptic effect of the drug is combined with sedative activity and anxiolytic effect. Unlike tricyclic antidepressants, it does not have anticholinergic properties, does not affect the activity of MAO, and does not have a cardiotoxic effect.
Pharmacokinetics
Suction
Quickly and completely absorbed from the gastrointestinal tract. Bioavailability – about 80%. Tmax after taking tablets – 2 hours.
In Azafen MB tablets, the active substance is contained in a special carrier matrix, which ensures the gradual release of pipofezin in the gastrointestinal tract. The released pipofezin is quickly and almost completely absorbed from the gastrointestinal tract. With a single oral dose of Azafen MB 150 mg tablet, the Cmax of pipofezin in the blood is achieved after 3-4 hours and is 111 ng/ml.
Distribution and metabolism
Plasma protein binding – 90%.
It undergoes significant biotransformation in the liver with the formation of inactive metabolites.
An in vitro study showed that pipofezin is not a substrate of the isoenzymes CYP2C9, CYP2C19, CYP2D6 and CYP3A4, but is predominantly metabolized under the influence of CYP1A2.
Removal
T1/2 after taking tablets – 16 hours, T1/2 after taking modified-release tablets – 9 hours. Excreted from the body mainly by the kidneys.
The residence time of the drug in the body (MRT) is an average of 13.4 hours (from 10 to 20 hours). With repeated doses, fluctuations in the concentration of the drug in the blood plasma in the interval between two doses are smoothed out. It is excreted from the body primarily by the kidneys.
Special instructions
Special instructions
After transferring from therapy with MAO inhibitors to Azafen, an interval of 1-2 weeks is required.
During the treatment period you should refrain from drinking alcohol. Any depressive disorder itself increases the risk of suicide. Therefore, during treatment, patients should be monitored for early detection of disturbances or changes in behavior, as well as suicidality.
Influence on the performance of potentially hazardous activities that require special attention and speed of psychomotor reactions
Due to a possible decrease in concentration during the treatment period, you should refrain from engaging in potentially hazardous activities that require increased attention and speed of psychomotor reactions (driving vehicles, working with moving mechanisms, working as a dispatcher and operator, etc.).
Active ingredient
Active ingredient
Pipofezin
Composition
Composition
Active substance:
pipofezin dihydrochloride monohydrate 25 mg
excipients:
potato starch – 4 mg;
colloidal silicon dioxide (Aerosil) – 1.75 mg;
MCC – 45 mg;
lactose monohydrate – 22 mg;
povidone (low molecular weight medical PVP) – 1.25 mg;
magnesium stearate – 1 mg
Contraindications
Contraindications
Hypersensitivity to the main and/or auxiliary components of the drug; severe liver and/or kidney failure; simultaneous use of MAO inhibitors; pregnancy; lactation period; children under 18 years of age (experience of medical use in children is limited).
Side Effects
Side Effects
Headache, dizziness, nausea, vomiting, allergic reactions.
At the beginning of therapy, weakness, drowsiness, impaired concentration, and dry mouth may appear, which are leveled out without additional treatment.
Interaction
Interaction
Enhances the effects of ethanol, antihistamines and other central nervous system depressants and anticoagulants.
Reduces the effectiveness of antiepileptic drugs.
An in vitro study showed that Azafen is not an inhibitor or inducer of the isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6 and CYP3A4, so it is unlikely that Azafen interacts with drugs that are substrates of these isoenzymes.
Fluvoxamine, propafenone, mexiletine, ciprofloxacin (inhibitors of the CYP1A2 isoenzyme) may increase the concentration of Azafen in the blood plasma.
Overdose
Overdose
No information available.
Storage conditions
Storage conditions
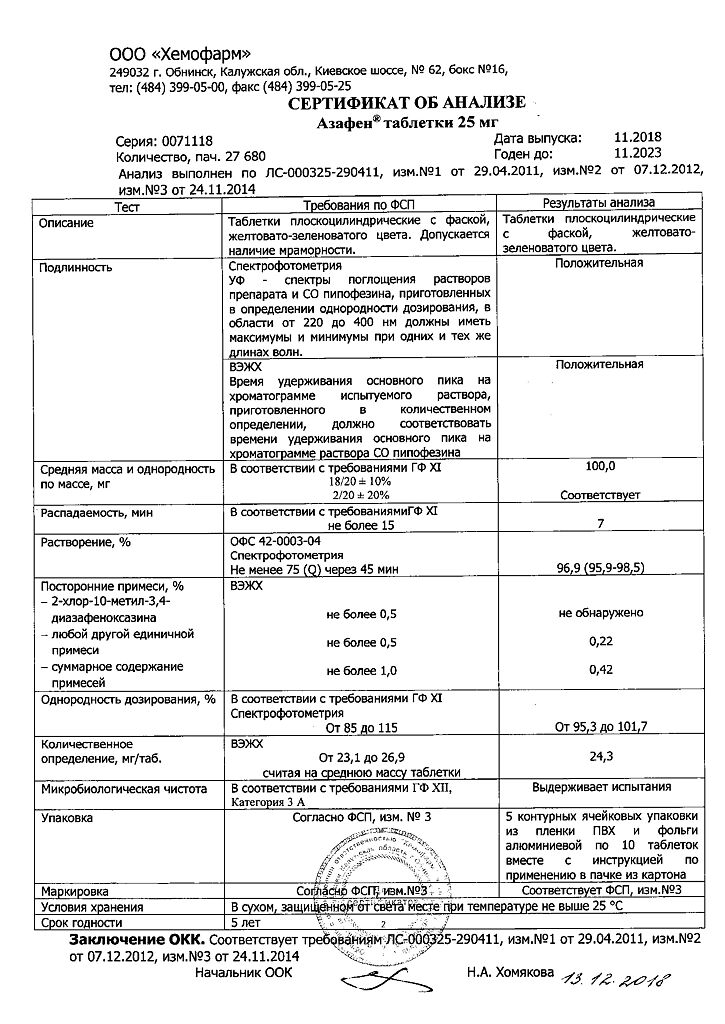
List B. Store in a dry place, protected from light, at a temperature not exceeding 25°C.
Shelf life
Shelf life
5 years.
Manufacturer
Manufacturer
Hemofarm LLC, Russia
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | List B. Store in a dry place, protected from light, at a temperature not exceeding 25 ° C. |
| Manufacturer | Chemopharm LLC, Russia |
| Medication form | pills |
| Brand | Chemopharm LLC |
Related products
Buy Azafen, tablets 25 mg 50 pcs with delivery to USA, UK, Europe and over 120 other countries.