No products in the cart.
Ariprizole, tablets 10 mg 30 pcs
€145.02 €120.85
Description
Schizophrenia, Mental Disorders, Manic Depressive Psychosis
- Schizophrenia: acute attacks and maintenance therapy
- Bipolar disorder type I: Manic episodes and maintenance therapy to prevent relapses in patients with type I bipolar disorder who have recently had a manic or mixed episode
- Addition to antidepressant therapy for major depressive disorder
Indications
Indications
Schizophrenia: acute attacks and maintenance therapy
Bipolar I disorder: manic episodes and maintenance therapy to prevent relapse in patients with bipolar I disorder who have recently had a manic or mixed episode
Adjunct to lithium or valproic acid therapy for the treatment of manic or mixed episodes of bipolar I disorder with or without psychotic symptoms and maintenance therapy to prevent relapse in patients with bipolar I disorder
Adjunct to antidepressant therapy for major depressive disorder
Special instructions
Special instructions
The therapeutic effect of antipsychotic drugs develops over several days to several weeks. During this period, it is necessary to monitor the patient’s condition.
Suicidal attempts.
The phenomenon of suicidal behavior is characteristic of psychosis and mood swings, in some cases it is observed immediately after the start or change of treatment with antipsychotic drugs, including treatment with aripiprazole. When treating with antipsychotic drugs, it is necessary to monitor patients at increased risk. The results of one epidemiological study showed that patients with schizophrenia or bipolar disorder did not experience an increased risk of suicidality when treated with aripiprazole compared with other antipsychotic drugs. There are insufficient clinical data to assess this risk in younger patients (<18 years of age), but there is evidence to suggest that the risk remains after 4 weeks of treatment with antipsychotic drugs, including aripiprazole.
Cardiovascular diseases.
Aripiprazole should be used with caution in patients with cardiovascular disease (myocardial infarction, coronary artery disease, heart failure, history of cardiac conduction disorders), cerebrovascular accidents, conditions predisposing to arterial hypotension (dehydration, hypovolemia, therapy with antihypertensive drugs) or hypertension, including essential or malignant.
Cases of venous thromboembolism have been reported with the use of antipsychotic drugs. Since patients treated with antipsychotic drugs often have acquired risk factors for the development of venous thromboembolism, it is necessary to determine all possible risk factors for the development of venous thromboembolism before and during treatment with Ariprizole® with preventive measures.
Conduction disorders.
In clinical studies of aripiprazole, the incidence of QT prolongation was comparable to the placebo group. Aripiprazole, like other antipsychotic drugs, should be used with caution in patients with a family history of QT prolongation.
Tardive dyskinesia.
In clinical studies lasting less than 1 year, infrequent cases of dyskinesia requiring urgent treatment were observed during treatment with aripiprazole. If a patient develops signs and symptoms of tardive dyskinesia while being treated with Ariprizole, dose reduction or discontinuation of treatment should be considered.
Symptoms of dyskinesia may temporarily increase or even appear for the first time after discontinuation of therapy.
Other extrapyramidal disorders.
Akathisia and parkinsonism were observed in clinical studies of aripiprazole in children. If signs and symptoms of other extrapyramidal disorders occur, consider reducing the aripiprazole dose and monitor the patient.
Neuroleptic malignant syndrome (NMS).
Neuroleptic malignant syndrome is a potentially life-threatening constellation of symptoms associated with the use of antipsychotic drugs. In clinical studies, rare cases of NMS were observed during treatment with aripiprazole, which is manifested by hyperpyrexia, muscle rigidity, mental disturbances and instability of the autonomic nervous system (irregular pulse and blood pressure, tachycardia, sweating, cardiac arrhythmia). In addition, in some cases, increased CPK activity, myoglobinuria (rhabdomyolysis) and acute renal failure occur. If symptoms of NMS or unexplained fever occur, all antipsychotics, including Ariprizole®, should be discontinued.
Cramps.
In clinical studies, infrequent cases of seizures were observed during treatment with aripiprazole. Therefore, aripiprazole should be used with caution in patients with a history of seizures and a risk of developing them.
Psychoses associated with senile dementia.
In three placebo-controlled clinical trials of aripiprazole in elderly patients (mean age 82.4 years, age range 56-99 years) with psychosis due to Alzheimer’s disease, an increased risk of death was observed compared with the placebo group. The mortality rate with aripiprazole was 3.5% compared with 1.7% in the placebo group. Although the causes of death varied, the underlying causes of most deaths were either cardiovascular disorders (including heart failure, sudden death) or infection (including pneumonia).
During these same clinical studies, cerebrovascular adverse reactions (including stroke, transient ischemic attack), including death, were reported in elderly patients (mean age 84 years, age range 78-88 years). Overall, 1.3% of patients treated with aripiprazole experienced cerebrovascular adverse events compared with 0.6% of patients treated with placebo. This difference was not statistically significant. However, one of these fixed-dose studies of aripiprazole found a significant dose relationship with cerebrovascular adverse reactions.
Ariprizole® is not recommended for use in patients with psychosis due to dementia.
Hyperglycemia and diabetes mellitus.
Hyperglycemia, in some cases severe and accompanied by ketoacidosis or hyperosmolar coma with a fatal outcome, has been noted in patients taking atypical antipsychotics. The relationship between atypical antipsychotics and hyperglycemic-type disorders remains unclear. In clinical studies of aripiprazole, there was no significant difference in the incidence of adverse reactions involving hyperglycemia (including diabetes) or changes in laboratory glycemic values compared with the placebo group.
Patients diagnosed with diabetes mellitus while taking atypical antipsychotics should regularly monitor their blood glucose concentrations.
Patients who have risk factors for developing diabetes mellitus (obesity, a family history of diabetes mellitus) when taking atypical antipsychotics should have their blood glucose levels determined at the beginning of the course and periodically while taking the drug. In patients taking atypical antipsychotics, constant monitoring of symptoms of hyperglycemia (increased thirst, frequent urination, polyphagia, weakness) is necessary. Particular attention should be paid to patients with diabetes mellitus and risk factors for its development.
Hypersensitivity.
As with other medications, hypersensitivity reactions in the form of allergic symptoms may occur when taking aripiprazole.
Increase in body weight.
Weight gain is commonly observed in patients with schizophrenia and bipolar mania due to the development of comorbidities, the use of antipsychotic drugs that cause weight gain, and unhealthy lifestyle choices that can lead to acute complications. Case reports of weight gain have been received during the post-marketing period in patients taking aripiprazole. Typically, these adverse reactions were observed in patients with significant risk factors, such as diabetes mellitus, thyroid disease or pituitary adenoma. In clinical studies, aripiprazole did not cause clinically significant weight gain.
In clinical studies of adolescent patients with bipolar mania treated with aripiprazole, body weight increased after 4 weeks of treatment. Continuous monitoring of body weight is necessary in adolescent patients with bipolar mania. If weight gain is clinically significant, the dose of aripiprazole should be reduced.
Dysphagia.
When using antipsychotics, there have been cases of disturbances in esophageal peristalsis and, as a consequence, aspiration pneumonia. The drug should be prescribed with caution to patients with risk factors for developing aspiration pneumonia.
Pathological attraction to gambling.
Post-marketing reports of pathological gambling have been reported in patients taking aripiprazole, regardless of whether these patients had a history of pathological gambling. Patients with a history of pathological gambling may be at increased risk of developing this disorder and should be closely monitored while using aripiprazole.
Lactose.
The drug Ariprizole® contains lactose, so it is not recommended for patients with rare hereditary diseases associated with galactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Patients with concomitant attention deficit hyperactivity disorder (ADHD).
Despite the high incidence of co-occurrence of bipolar I disorder and ADHD, there are very limited data on the safety of the concomitant use of aripiprazole and psychostimulants, so caution should be exercised if they are used together.
Impact on the ability to drive vehicles and machinery
As with the use of other antipsychotics, when prescribing aripiprazole, the patient should be warned about the dangers of working with moving mechanisms and driving a car.
Active ingredient
Active ingredient
Aripiprazole
Composition
Composition
One tablet contains:
Active substance:
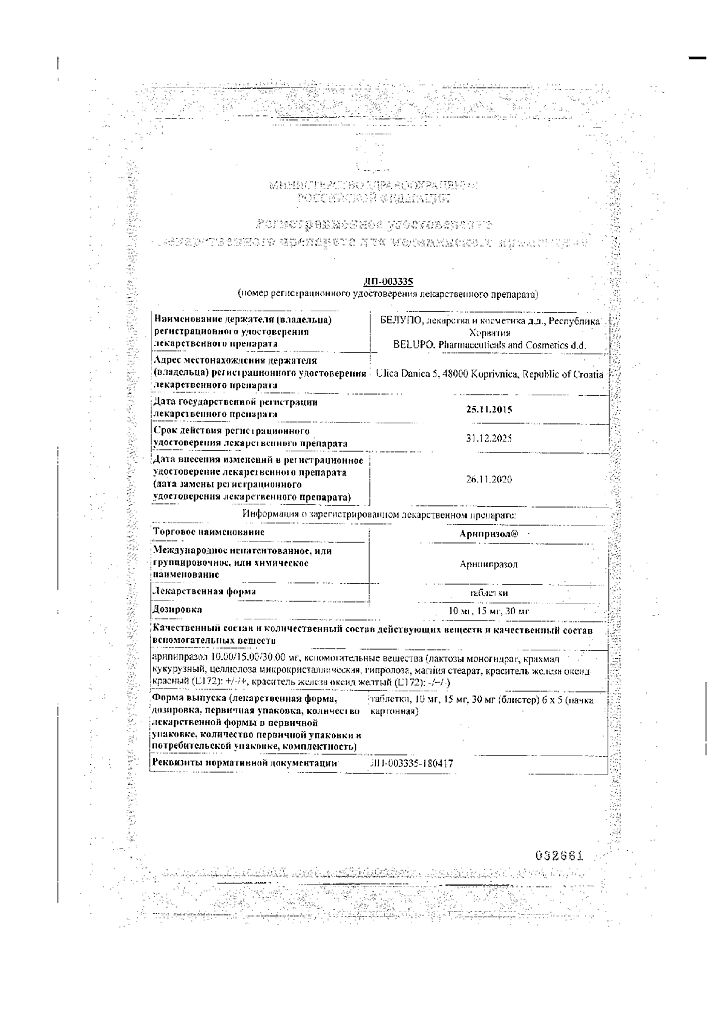
aripiprazole 10.00 / 15.00 / 30.00 mg.
Excipients:
lactose monohydrate – 61.23 / 91.53 / 183.69 mg,
corn starch – 10.45 / 15.675 / 31.35 mg,
microcrystalline cellulose – 10.45 / 15.675 / 31.35 mg,
hyprolose – 1.90 / 2.85 / 5.70 mg,
magnesium stearate – 0.95 / 1.425 / 2.85 mg,
red iron oxide dye (E172) (for dosages of 10 mg and 30 mg) – 0.02 / 0.06 mg,
iron oxide yellow dye (E172) (for a dosage of 15 mg) – 0.345 mg.
Contraindications
Contraindications
Hypersensitivity to aripiprazole or to any component of the drug;
age under 18 years;
lactase deficiency, rare hereditary galactosemia, glucose-galactose malabsorption.
With caution
In patients with cardiovascular diseases (coronary heart disease or previous myocardial infarction, heart failure and conduction disorders), cerebrovascular diseases and conditions predisposing to arterial hypotension (dehydration, hypovolemia and taking antihypertensive drugs) due to the possibility of developing orthostatic hypotension; in patients with seizures or suffering from diseases in which seizures are possible; in patients with an increased risk of hyperthermia (for example, during intense physical activity, overheating, taking m-anticholinergic drugs, dehydration due to the ability of antipsychotics to disrupt thermoregulation); in patients with an increased risk of aspiration pneumonia due to the risk of impaired motor function of the esophagus and aspiration; in obese patients and with a family history of diabetes; in patients at high risk of suicide (psychotic illnesses, bipolar disorders, major depressive disorder); in persons aged 18-24 years due to the risk of developing suicidal behavior.
Side Effects
Side Effects
The most commonly reported side effects in placebo-controlled clinical trials were akathisia and nausea, each of which occurred in more than 3% of patients treated with aripiprazole.
The following side effects are more common (≥ 1/100) compared to placebo or are identified as possible clinically significant side effects (*).
The following classification is used to indicate the frequency of side effects: often (˃ 1/100 and ˂ 1/10) and infrequently (˃ 1/1000 and ˂ 1/100):
Mental disorders:
often – restlessness, insomnia, anxiety;
infrequently – depression*, increased sexuality.
Nervous system disorders:
often – extrapyramidal disorders, akathisia, tremor, dizziness, drowsiness, sedation, headache.
Visual disorders:
often – blurred vision; infrequently – diplopia.
Cardiovascular system disorders:
uncommon – tachycardia*, orthostatic hypotension*.
Gastrointestinal disorders:
often – dyspepsia, vomiting, nausea, constipation, hypersecretion of saliva.
General violations:
often – fatigue.
Post-marketing surveillance
The following adverse reactions have been reported during post-marketing studies. The incidence of adverse reactions is considered unknown because it cannot be estimated based on available data.
From the hematopoietic organs: leukopenia, neutropenia, thrombocytopenia.
From the immune system: allergic reactions (anaphylactic shock, angioedema, including inflammation of the tongue, swelling of the tongue, swelling of the face, itching or urticaria).
From the endocrine system: hyperglycemia, diabetes mellitus, diabetic ketoacidosis, diabetic hyperosmolar coma.
Metabolism and nutrition: weight gain, weight loss, anorexia, hyponatremia.
Mental disorders: agitation, nervousness, pathological gambling, attempted suicide, suicidal thoughts, completed suicide.
From the nervous system: speech disorder, neuroleptic malignant syndrome, seizures, serotonin syndrome.
From the cardiovascular system: prolongation of the QT interval, ventricular arrhythmia, sudden death, cardiac arrest, tachycardia, bradycardia, syncope, increased blood pressure, thromboembolism (including pulmonary embolism and deep vein thrombosis).
From the respiratory system: oropharyngeal spasm, laryngospasm, aspiration pneumonia.
From the gastrointestinal tract: pancreatitis, dysphagia, heaviness in the abdomen, diarrhea.
From the liver and biliary tract: liver failure, jaundice, hepatitis, increased activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
From the skin and subcutaneous tissues: rash, photosensitivity, alopecia, hyperhidrosis.
From the musculoskeletal system: rhabdomyolysis, myaglia, muscle weakness.
From the urinary system: urinary incontinence, urinary retention.
From the reproductive system: priapism.
General disorders: disturbances in temperature regulation (including hypothermia, pyrexia), chest pain, peripheral edema, increased creatine phosphokinase (CPK) activity, increased blood glucose levels, fluctuations in blood glucose levels, increased glycohemoglobin levels.
Interaction
Interaction
Aripiprazole may enhance the effect of antihypertensive drugs because it has an antagonistic effect on alpha1-adrenergic receptors.
The mechanism of action of aripiprazole is associated with an effect on the central nervous system; this must be taken into account when used together with other drugs that have a central effect.
Caution should be exercised during the simultaneous use of ariprizole and drugs that cause prolongation of the QT interval or disrupt electrolyte balance.
Overdose
Overdose
Clinical studies have described cases of accidental or intentional overdose of aripiprazole with a single dose of up to 1260 mg, which were not accompanied by death.
Symptoms: lethargy, increased blood pressure, drowsiness, tachycardia, loss of consciousness, nausea, vomiting, diarrhea. In hospitalized patients, no clinically significant changes in basic physiological parameters, laboratory parameters and ECG were detected.
Cases of aripiprazole overdose in children (taking up to 195 mg) without death have been described. Potentially dangerous symptoms of overdose are drowsiness, extrapyramidal disturbances and transient loss of consciousness.
Treatment: monitoring vital signs, ECG to detect possible arrhythmia, supportive care, airway management, oxygenation, effective ventilation, activated charcoal, symptomatic treatment, careful medical observation until all symptoms disappear. There are no data on the use of hemodialysis for aripiprazole overdose; a beneficial effect of this method is unlikely, since aripiprazole is not excreted unchanged by the kidneys and is largely bound to plasma proteins.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Belupo, medicines and cosmetics d.d., Croatia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
| Manufacturer | Belupo,medicines and cosmetics d.d., Croatia |
| Medication form | pills |
| Brand | Belupo,medicines and cosmetics d.d. |
Other forms…
Related products
Buy Ariprizole, tablets 10 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.