No products in the cart.
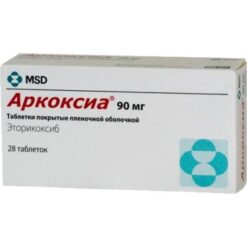
Arcoxia, 90 mg 7 pcs
€16.65 €13.87
Description
Pharmgroup:
NPVS. A highly selective COX-2 inhibitor.
Pharmic action:
Arcoxia is an NSAID. A selective COX-2 inhibitor, in therapeutic concentrations blocks the formation of prostaglandins and has anti-inflammatory, analgesic and antipyretic effects. Selective inhibition of COX-2 is accompanied by a decrease in the severity of clinical symptoms associated with the inflammatory process, with no effect on platelet function and gastrointestinal mucosa.
Etoricoxib has a dose-dependent effect of COX-2 inhibition with no effect on COX-1 when used in daily doses up to 150 mg. Arcoxia® has no effect on the production of prostaglandins in the gastric mucosa and on bleeding time. In the conducted studies, no reduction in arachidonic acid and collagen-induced platelet aggregation was observed.
Pharmacokinetics:
absorption
After oral administration it is quickly absorbed from the GI tract. Bioavailability when taken orally is about 100%. After oral administration in adults on an empty stomach at a dose of 120 mg, Cmax is 3.6 mcg/ml, time to reach Cmax -1 hour after administration.
Eat intake has no significant effect on the intensity and absorption rate of etoricoxib when taken in a dose of 120 mg. At the same time there is a decrease of Cmax values by 36% and increase in Tmax by 2 h.
Antacid intake does not affect pharmacokinetics of the drug.
Distribution
The geometric mean AUC0-24 was 37.8 µg × h/mL. The pharmacokinetics of etoricoxib within therapeutic doses is linear.
Binding to plasma proteins exceeds 92%. Vd in equilibrium is about 120 l. Etoricoxib penetrates through the placental and blood-brain barrier.
Metabolism
Intensively metabolized in the liver, with participation of cytochrome P450 isoenzyme (CYP) and formation of 6-hydroxymethyl etoricoxib. Five metabolites of etoricoxib have been detected, the main ones being 6-hydroxymethyl-etoricoxib and its derivative, 6-carboxy acetyl-etoricoxib. The main metabolites have no effect on COX-1 and are inactive or inactive against COX-2.
Elimation
Elimation of etoricoxib occurs as metabolites by the kidneys. Less than 1% of the drug is excreted unchanged in the urine.
In a single intravenous administration of a labeled radioactive drug containing etoricoxib at a dose of 25 mg to healthy volunteers, it has been demonstrated that 70% of the drug is excreted through the kidneys, 20% – through the intestine, mostly as metabolites. Less than 2% was found unchanged.
The equilibrium state is reached after 7 days at a daily dose of 120 mg, with a cumulation factor of about 2, which corresponds to a T1/2 of about 22 hours. Plasma clearance is approximately 50 ml/min.
Pharmacokinetics in special clinical cases
There are no pharmacokinetic differences in men and women.
The pharmacokinetics in the elderly (65 years and older) are comparable to those in the young, and there is no need to adjust the dose of the drug in the elderly.
Racial differences do not affect the pharmacokinetic parameters of etoricoxib.
In patients with mild hepatic impairment (Child-Pugh score of 5-6), a single dose of etoricoxib at a dose of 60 mg/day was accompanied by a 16% increase in AUC compared to healthy subjects.
In patients with moderate hepatic impairment (Child-Pugh score 7-9) who received the drug in a dose of 60 mg every other day, the AUC was the same as in healthy subjects who took the drug daily in the same dose.
There are no data from clinical and pharmacokinetic studies in patients with severe hepatic impairment (more than 9 points on the Child-Pugh scale).
The pharmacokinetic parameters of a single dose of etoricoxib 120 mg in patients with moderate to severe renal impairment and with end-stage chronic renal disease (CKD) on hemodialysis were not significantly different from those in healthy subjects. Hemodialysis had little effect on excretion (dialysis clearance was about 50 ml/min).
The pharmacokinetic parameters of etoricoxib in children younger than 12 years have not been studied. In comparative pharmacokinetic studies we obtained comparable data when using etoricoxib in the group of adolescents (from 12 to 17 years old) with body weight of 40-60 kg at a dose of 60 mg/day, in a similar age group and with body weight over 60 kg – 90 mg/day, and in adults when taking 90 mg/day.
Indications
Indications
Symptomatic treatment of the following diseases and conditions:
Rheumatoid arthritis.
Osteoarthritis.
Ankylosing spondylitis.
Pain and inflammatory symptoms associated with acute gouty arthritis.
Treatment of moderate and severe acute pain after dental surgery.
Pharmacological effect
Pharmacological effect
Pharmgroup:
NSAIDs. Highly selective COX-2 inhibitor.
Pharmaceutical action:
Arcoxia is an NSAID. A selective COX-2 inhibitor, in therapeutic concentrations, blocks the formation of prostaglandins and has anti-inflammatory, analgesic and antipyretic effects. Selective inhibition of COX-2 is accompanied by a decrease in the severity of clinical symptoms associated with the inflammatory process, while there is no effect on platelet function and the gastrointestinal mucosa.
Etoricoxib has a dose-dependent effect of inhibiting COX-2, without affecting COX-1 when used in a daily dose of up to 150 mg. Arcoxia® does not affect the production of prostaglandins in the gastric mucosa and the bleeding time. In the studies conducted, there was no decrease in arachidonic acid levels and platelet aggregation caused by collagen.
Pharmacokinetics:
Suction
After oral administration, it is quickly absorbed from the gastrointestinal tract. Bioavailability when taken orally is about 100%. After taking the drug by adults on an empty stomach at a dose of 120 mg, Cmax is 3.6 mcg/ml, the time to reach Cmax is 1 hour after administration.
Food intake does not have a significant effect on the severity and rate of absorption of etoricoxib when taken at a dose of 120 mg. At the same time, there is a decrease in Cmax values by 36% and an increase in Tmax by 2 hours.
Taking antacids does not affect the pharmacokinetics of the drug.
Distribution
The geometric mean AUC0-24 was 37.8 µg x h/ml. The pharmacokinetics of etoricoxib within therapeutic doses is linear.
Plasma protein binding exceeds 92%. Vd at equilibrium is about 120 l. Etoricoxib penetrates the placental and blood-brain barrier.
Metabolism
Intensively metabolized in the liver, with the participation of the cytochrome P450 isoenzyme (CYP) and the formation of 6-hydroxymethyl etoricoxib. Five metabolites of etoricoxib were discovered, the main ones being 6-hydroxymethyl-etoricoxib and its derivative 6-carboxy-acetyl-etoricoxib. The main metabolites do not affect COX-1 and are completely inactive or have little activity against COX-2.
Removal
Etoricoxib is excreted as metabolites by the kidneys. Less than 1% of the drug is excreted unchanged in the urine.
With a single intravenous administration of a radiolabeled drug containing etoricoxib at a dose of 25 mg to healthy volunteers, it was demonstrated that 70% of the drug was excreted through the kidneys, 20% through the intestines, mainly in the form of metabolites. Less than 2% was found unchanged.
The equilibrium state is achieved after 7 days with a daily dose of 120 mg, with a cumulation coefficient of about 2, which corresponds to T1/2 – about 22 hours. Plasma clearance is approximately 50 ml/min.
Pharmacokinetics in special clinical situations
There are no pharmacokinetic differences between men and women.
Pharmacokinetics in the elderly (65 years and older) are comparable to those in the young, and there is no need to adjust the dose of the drug in the elderly.
Racial differences do not affect the pharmacokinetic parameters of etoricoxib.
In patients with minor liver dysfunction (5-6 points on the Child-Pugh scale), a single dose of etoricoxib at a dose of 60 mg/day. was accompanied by an increase in AUC by 16% compared to healthy individuals.
In patients with moderate liver dysfunction (7-9 points on the Child-Pugh scale) taking the drug at a dose of 60 mg every other day, the AUC value was the same as in healthy individuals taking the drug daily at the same dose.
Data from clinical and pharmacokinetic studies in patients with severe liver dysfunction (more than 9 points on the Child-Pugh scale) are not available.
The pharmacokinetic parameters of a single dose of etoricoxib 120 mg in patients with moderate to severe renal failure and end-stage chronic renal failure (ESRD) on hemodialysis did not differ significantly from those in healthy individuals. Hemodialysis had little effect on excretion (dialysis clearance – about 50 ml/min).
The pharmacokinetic parameters of etoricoxib have not been studied in children under 12 years of age. In comparative pharmacokinetic studies, comparable data were obtained when using etoricoxib in a group of adolescents (from 12 to 17 years old) with a body weight of 40-60 kg at a dose of 60 mg/day, in a similar age group and with a body weight of more than 60 kg – 90 mg/day, and in adults when taking 90 mg/day.
Special instructions
Special instructions
Taking the drug Arcoxia® requires careful monitoring of blood pressure. When prescribing the drug, all patients should have their blood pressure monitored during the first two weeks of treatment and periodically thereafter.
Liver and kidney function tests should also be regularly monitored.
If the level of liver transaminases increases by 3 times or more relative to ULN, the drug should be discontinued.
Given the increasing risk of developing undesirable effects with increasing duration of use, it is necessary to periodically evaluate the need to continue taking the drug and the possibility of reducing the dose.
The drug should not be used simultaneously with other NSAIDs.
The shell of Arcoxia® contains lactose in small quantities, which should be taken into account when prescribing the drug to patients with lactase deficiency.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions. Patients who have experienced episodes of dizziness, drowsiness or weakness should refrain from activities that require concentration.
Active ingredient
Active ingredient
Etoricoxib
Composition
Composition
1 tablet contains:
Active substance:
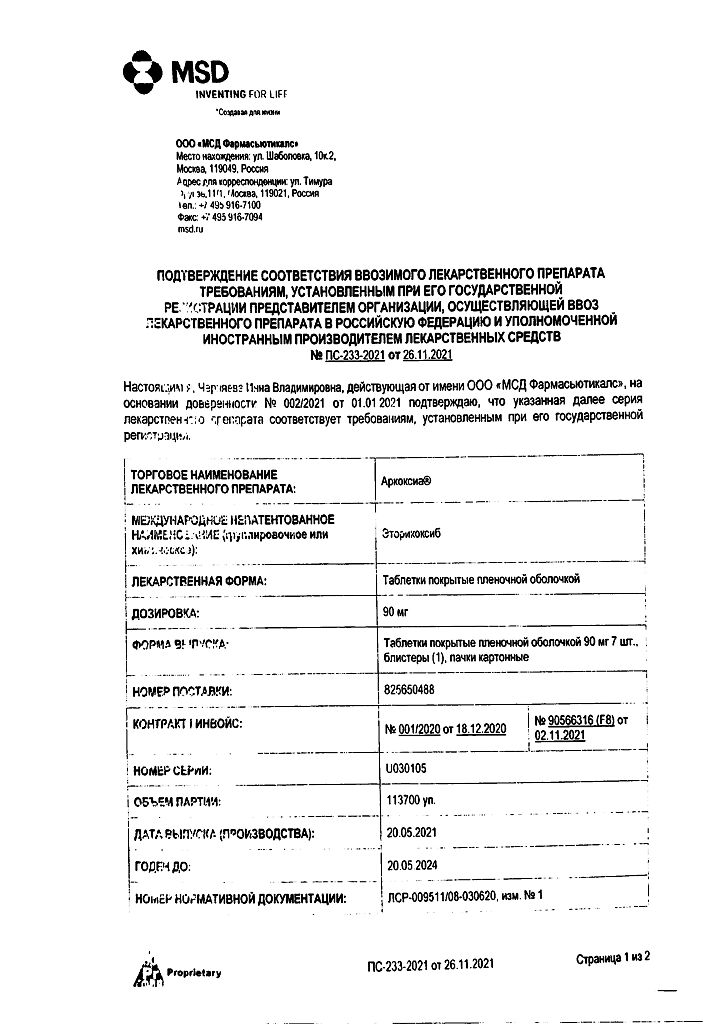
Etoricoxib 90 mg.
Excipients:
Calcium hydrophosphate;
Microcrystalline cellulose;
Croscarmellose sodium;
Magnesium stearate.
Shell composition:
Opadry II white 39K18305;
Carnauba wax.
Film shell composition:
Lactose monohydrate;
Hypromellose;
Titanium dioxide;
Triacetin.
Pregnancy
Pregnancy
The drug is contraindicated during pregnancy and lactation.
The use of the drug may adversely affect female fertility and is not recommended for women planning pregnancy.
Contraindications
Contraindications
Erosive and ulcerative changes in the mucous membrane of the stomach or duodenum, active gastrointestinal bleeding, cerebrovascular or other bleeding.
Complete or incomplete combination of bronchial asthma, recurrent nasal polyposis or paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including a history).
Inflammatory bowel diseases (Crohn’s disease, ulcerative colitis) in the acute phase.
Hemophilia and other bleeding disorders.
Severe heart failure (II-IV functional classes according to the NYHA classification).
Severe liver failure (more than 9 points on the Child-Pugh scale) or active liver disease.
Severe renal failure (creatinine clearance less than 30 ml/min), progressive kidney disease, confirmed hyperkalemia.
The period after coronary artery bypass surgery; peripheral arterial diseases, cerebrovascular diseases, clinically significant ischemic heart disease.
Persistent blood pressure values exceeding 140/90 mmHg. Art. with uncontrolled arterial hypertension.
Pregnancy.
Lactation period (breastfeeding).
Children’s age up to 16 years.
Hypersensitivity to any component of the drug.
With caution: use the drug in the presence of anamnestic data on the development of ulcerative lesions of the gastrointestinal tract, Helicobacter pylori infection, in the elderly, in patients who have been using NSAIDs for a long time, frequently drinking alcohol, with severe somatic diseases, dyslipidemia/hyperlipidemia, with diabetes mellitus, arterial hypertension, edema and fluid retention, smoking, in patients with creatinine clearance less than 60 ml/min, with concomitant therapy the following medications: anticoagulants (for example, warfarin), antiplatelet agents (for example, acetylsalicylic acid, clopidogrel), corticosteroids (for example, prednisolone), selective serotonin reuptake inhibitors (for example, citalopram, fluoxetine, paroxetine, sertraline).
Side Effects
Side Effects
The frequency of adverse reactions is presented in accordance with the following gradation:
Very common (≥10%).
Often (1-10%).
Uncommon (0.1-1%).
Rarely (0.01-0.1%).
Very rare (<0.01%, including isolated cases).
From the digestive system: often – epigastric pain, heartburn, nausea, diarrhea, dyspepsia, flatulence; uncommon – bloating, belching, increased peristalsis, constipation, dry oral mucosa, gastritis, ulcer of the gastric or duodenal mucosa, irritable bowel syndrome, esophagitis, ulcers of the oral mucosa, vomiting; very rarely – gastrointestinal ulcers (with bleeding or perforation), hepatitis.
From the nervous system: often – headache, dizziness, weakness; uncommon – taste disturbance, drowsiness, sleep disturbances, sensory disturbances, incl. paresthesia/hyperesthesia, anxiety, depression, concentration disorders; very rarely – hallucinations, confusion.
From the senses: infrequently – blurred vision, conjunctivitis, tinnitus, vertigo.
From the urinary system: infrequently – proteinuria; very rarely – renal failure, usually reversible when the drug is discontinued.
Allergic reactions: very rarely – anaphylactic/anaphylactoid reactions, including a pronounced decrease in blood pressure and shock.
From the cardiovascular system: often – palpitations, increased blood pressure; uncommon – hot flashes, cerebrovascular accident, atrial fibrillation, congestive heart failure, nonspecific ECG changes; myocardial infarction; very rarely – hypertensive crisis.
From the respiratory system: infrequently – cough, shortness of breath, nosebleeds; very rarely – bronchospasm.
Dermatological reactions: often – ecchymosis; infrequently – swelling of the face, itching, rash; very rarely – urticaria, Stevens-Johnson syndrome, Lyell’s syndrome.
Infectious complications: uncommon – gastroenteritis, infections of the upper respiratory tract, urinary tract.
From the musculoskeletal system: infrequently – muscle cramps, arthralgia, myalgia.
Metabolism: often – swelling, fluid retention; infrequently – changes in appetite, weight gain.
From laboratory tests: often – increased activity of liver transaminases; uncommon – increased nitrogen in the blood and urine, increased CPK activity, decreased hematocrit, decreased hemoglobin, hyperkalemia, leukopenia, thrombocytopenia, increased serum creatinine, increased uric acid; rarely – increased sodium in the blood serum.
Other: often – flu-like syndrome; infrequently – chest pain.
Interaction
Interaction
Pharmacodynamic interaction
In patients receiving warfarin, taking Arcoxia® at a dose of 120 mg/day was accompanied by an increase of approximately 13% in MHO and prothrombin time. In patients receiving warfarin or similar drugs, MHO levels should be monitored when initiating therapy or changing the Arcoxia® dosage regimen, especially in the first few days.
There are reports that non-selective NSAIDs and selective COX-2 inhibitors may weaken the hypotensive effect of ACE inhibitors. This interaction should be taken into account when treating patients taking Arcoxia® concomitantly with ACE inhibitors. In patients with impaired renal function (for example, with dehydration or in old age), such a combination may aggravate renal failure.
Arcoxia® can be used simultaneously with acetylsalicylic acid in low doses intended for the prevention of cardiovascular diseases. However, simultaneous administration of acetylsalicylic acid in low doses and Arcoxia® may lead to an increase in the incidence of gastrointestinal ulcers and other complications compared to taking Arcoxia® alone. After reaching a steady state, taking etoricoxib at a dose of 120 mg 1 time / day does not affect the antiplatelet activity of acetylsalicylic acid in low doses (81 mg / day). The drug does not replace the preventive effect of acetylsalicylic acid in cardiovascular diseases.
Cyclosporine and tacrolimus increase the risk of nephrotoxicity while taking Arcoxia®.
Pharmacokinetic interaction
There is evidence that non-selective NSAIDs and selective COX-2 inhibitors may increase plasma lithium concentrations. This interaction should be taken into account when treating patients taking Arcoxia® concomitantly with lithium.
Two studies examined the effects of Arcoxia® at doses of 60, 90 and 120 mg once a day for seven days in patients receiving methotrexate at a dose of 7.5 to 20 mg once a week for rheumatoid arthritis. Arcoxia® at a dose of 60 and 90 mg had no effect on the plasma concentration (according to AUC) and renal clearance of methotrexate. In one study, Arcoxia® at a dose of 120 mg had no effect on the plasma concentration (AUC) and renal clearance of methotrexate. In another study, Arcoxia® at a dose of 120 mg increased the plasma concentration of methotrexate by 28% (based on AUC) and decreased the renal clearance of methotrexate by 13%. When prescribing Arcoxia® at doses above 90 mg/day and methotrexate simultaneously, monitoring for the possible occurrence of toxic effects of methotrexate should be carried out.
Oral contraceptives: Taking Arcoxia® at a dose of 120 mg with oral contraceptives containing 35 mcg ethinyl estradiol and 0.5 to 1 mg norethindrone for 21 days, simultaneously or 12 hours apart, increases the steady-state AUC0-24 of ethinyl estradiol by 50-60%. However, norethisterone concentrations usually do not increase to a clinically significant extent. This increase in ethinyl estradiol concentrations should be taken into account when selecting the appropriate oral contraceptive for concomitant use with Arcoxia®. This fact may lead to an increased incidence of thromboembolism due to increased exposure to ethinyl estradiol. No significant pharmacokinetic interaction with GCS was detected.
Etoricoxib does not affect AUC0-24 at steady state or digoxin elimination. However, etoricoxib increases Cmax (by an average of 33%), which may be important in the development of digoxin overdose.
Co-administration of Arcoxia® and rifampicin (a powerful inducer of hepatic metabolism) leads to a 65% decrease in plasma etoricoxib AUC. This interaction should be taken into account when Arcoxia® is co-administered with rifampicin.
Antacids and ketoconazole (a strong CYP3A4 inhibitor) do not have a clinically significant effect on the pharmacokinetics of Arcoxia®.
Overdose
Overdose
Overdose of Arcoxia® was not reported in clinical trials. In clinical trials, a single dose of Arcoxia® in a single dose of up to 500 mg or repeated doses of up to 150 mg/day for 21 days did not cause significant toxic effects.
Symptoms: in case of an overdose of the drug, undesirable effects from the gastrointestinal tract, cardiovascular system and kidneys may occur.
Treatment: carry out symptomatic therapy. Etoricoxib is not eliminated by hemodialysis; elimination of the drug by peritoneal dialysis has not been studied.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature of 18–22 °C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Rovi Pharma Industrial Services S.A., Spain
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | In a dry, light-protected place at 18-22 °C. |
| Manufacturer | Rovi Pharma Industrial Services S.A., Spain |
| Medication form | pills |
| Brand | Rovi Pharma Industrial Services S.A. |
Other forms…
Related products
Buy Arcoxia, 90 mg 7 pcs with delivery to USA, UK, Europe and over 120 other countries.