No products in the cart.
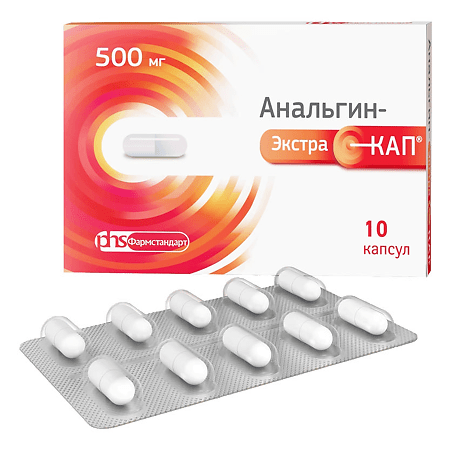
Analgin ExtraCap, 500 mg capsules 10 pcs
€3.89 €3.46
Description
Analgesic non-narcotic.
It has analgesic, antipyretic and mild anti-inflammatory effects, it is a derivative of pyrazolone.
Pharmacodynamics
The mechanism of action is not fully understood. According to studies, metamizole and its active metabolite (4Y-methylaminoantipyrine) have a central and peripheral mechanism of action. It nonselectively inhibits cyclooxygenase and reduces the formation of prostaglandins from arachidonic acid.
Pharmacokinetics:
Indications
Indications
Severe acute or chronic pain syndrome due to injuries and postoperative pain syndrome, colic, cancer and other conditions where other therapeutic measures are contraindicated.
Fever resistant to other treatments.
Pharmacological effect
Pharmacological effect
Analgesic non-narcotic drug.
It has analgesic, antipyretic and weak anti-inflammatory effects, and is a derivative of pyrazolone.
Pharmacodynamics
The mechanism of action is not fully understood. According to research results, metamizole and its active metabolite (4N-methylaminoantipyrine) have a central and peripheral mechanism of action. Non-selectively inhibits cyclooxygenase and reduces the formation of prostaglandins from arachidonic acid.
Pharmacokinetics:
Special instructions
Special instructions
The drug contains a pyrazolone derivative – metamizole sodium, which can rarely cause life-threatening shock and agranulocytosis (see section “Side effects”). Patients who experience anaphylactoid reactions in response to the use of metamizole sodium are also at risk of developing them in response to the use of other non-narcotic analgesics/NSAIDs.
Patients who experience anaphylactic or other immune-mediated reactions (eg, agranulocytosis) in response to the use of metamizole sodium are also at risk of developing them in response to the use of other pyrazolones and pyrazolidines.
Agranulocytosis
If signs of agranulocytosis or thrombocytopenia appear (see section “Side Effects”), the drug should be immediately discontinued and a general blood test (with determination of the leukocyte formula) should be performed. Discontinuation of therapy should not be delayed until laboratory results are available.
Pancytopenia
If pancytopenia develops, the drug must be immediately discontinued and the complete blood count must be monitored until its values return to normal (see section “Side Effects”). All patients should be advised to seek immediate medical attention if signs and symptoms suggestive of blood disorders occur during treatment (eg, general weakness, infections, persistent fever, bruising, bleeding, pallor).
Anaphylactic/anaphylactoid reactions
Before using metamizole sodium, it is necessary to conduct a thorough interview with the patient. If a risk of developing anaphylactoid reactions is identified, the use of the drug is allowed only after a thorough assessment of the possible risks and expected benefits.
If a decision is made to use metamizole sodium, the patient must be under strict medical supervision and emergency measures must be prepared.
An increased risk of developing hypersensitivity reactions to metamizole sodium is caused by the following conditions: analgesic bronchial asthma or intolerance to analgesics (urticaria-angioedema type) (see section “Contraindications”); bronchial asthma, especially accompanied by rhinosinusitis and nasal polyposis; chronic urticaria; intolerance to dyes (for example, tartrazine) or preservatives (for example, benzoates); alcohol intolerance, against the background of which, even when taking a small amount of alcoholic beverages, patients experience sneezing, lacrimation and severe redness of the face.
Alcohol intolerance may indicate previously unidentified analgesic bronchial asthma (see section “Contraindications))).
Anaphylactic shock may occur in susceptible patients, so special caution should be exercised in patients with asthma or atopy.
Severe skin reactions
Life-threatening skin reactions have been described with the use of metamizole sodium: Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). If signs of SJS or TEN appear (such as a progressive skin rash, often accompanied by blistering or ulceration of the mucous membrane), treatment with metamizole should be stopped immediately and never restarted. Patients should be educated about the signs and symptoms of these diseases. They should be carefully monitored for skin reactions, especially during the first weeks of treatment.
Isolated hypotensive reactions
Metamizole sodium may cause hypotensive reactions (see also section “Side effects”). These reactions may be dose-dependent. The risk of such reactions is also increased in: pre-existing arterial hypotension, decreased circulating blood volume or dehydration, unstable hemodynamics or acute circulatory impairment (for example, in patients with myocardial infarction or trauma), in patients with high fever.
In this regard, such patients should be subject to detailed diagnostics and careful monitoring. Preventive measures (eg, cardiovascular resuscitation) may be required to reduce the risk of hypotensive reactions. In patients in whom a decrease in blood pressure should be avoided at all costs (for example, with severe ischemic heart disease or significant cerebral artery stenosis), metamizole sodium should only be used with careful monitoring of hemodynamic parameters.
Abdominal pain
It is unacceptable to use the drug to relieve acute abdominal pain (until the cause is determined).
Impaired kidney or liver function
In patients with impaired renal or liver function, metamizole sodium should only be used after a strict assessment of the benefits and risks, taking all necessary precautions.
Impact on the ability to drive vehicles. Wed and fur.:
In the recommended dose range, the effect on concentration and speed of psychomotor reactions has not been established. When taking high doses, it is recommended to be careful when driving vehicles, operating machinery and engaging in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Metamizole sodium
Composition
Composition
1 capsule:
metamizole sodium 500 mg.
Excipients:
potato starch – 15.32 mg,
calcium stearate – 4.68 mg.
Composition of the capsule shell (body and cap):
titanium dioxide (E 171) – 2%,
gelatin up to 100%
Pregnancy
Pregnancy
Use during pregnancy and breastfeeding is contraindicated.
Pregnancy
Metamizole sodium penetrates the placental barrier. Data on the use of sodium metamazole during pregnancy are limited. According to the results of preclinical studies, the teratogenic effect of metamizole sodium in rats and rabbits was not detected; fetotoxicity was observed in high doses.
Since there are no adequate data on use in humans, metamizole sodium should not be taken in the first trimester of pregnancy; in the second trimester of pregnancy, metamizole sodium should only be used if the expected benefit to the mother outweighs the potential risk to the fetus.
Despite the fact that metamizole sodium weakly inhibits the synthesis of prostaglandins, the possibility of premature (intrauterine) closure of the arterial (Botalov) duct, as well as perinatal complications caused by impaired platelet aggregation in the mother or newborn cannot be excluded. Therefore, metamizole sodium is contraindicated in the third trimester of pregnancy.
Lactation period
Metamizole sodium metabolites pass into breast milk, therefore, when using the drug, as well as within 48 hours after taking the last dose, you must stop breastfeeding.
Contraindications
Contraindications
– Hypersensitivity to metamizole sodium and other pyrazolone derivatives, as well as to pyrazolidines, for example, phenylbutazone (including patients who have suffered agranulocytosis due to the use of these drugs), or other components of the drug;
– Analgesic bronchial asthma or intolerance to analgesics (urticaria-angioedema type), i.e. patients with bronchospasm or other forms of anaphylactoid reactions (for example, urticaria, rhinitis, angioedema) in response to the use of salicylates, paracetamol or non-steroidal anti-inflammatory drugs such as diclofenac, ibuprofen, indomethacin or naproxen;
– Impaired bone marrow hematopoiesis (for example, after cytostatic therapy) or diseases of the hematopoietic organs;
– Hereditary deficiency of glucose-6-phosphate dehydrogenase (hemolysis);
– Acute intermittent hepatic porphyria (risk of developing porphyria attacks);
– Pregnancy and breastfeeding period;
– Children under 15 years of age.
With caution:
– Arterial hypotension (systolic blood pressure below 100 mm Hg), decreased circulating blood volume, hemodynamic instability (myocardial infarction, multiple trauma, incipient shock), incipient heart failure, high fever (increased risk of a sharp decrease in blood pressure).
– Diseases in which a significant decrease in blood pressure may be of increased danger (patients with severe coronary heart disease and stenosis of the cerebral arteries).
– Chronic alcohol abuse.
– Bronchial asthma, especially in combination with concomitant polypous rhinosinusitis; chronic urticaria and other types of atopy (allergic diseases, in the development of which a hereditary predisposition to sensitization plays a significant role: hay fever, allergic rhinitis, etc.) (increased risk of developing anaphylactic/anaphylactoid reactions).
– Alcohol intolerance (reaction to even small amounts of certain alcoholic beverages with symptoms such as itching, lacrimation and severe redness of the face) (increased risk of developing anaphylactic/anaphylactoid reactions).
– Intolerance to dyes (for example, tartrazine) or preservatives (for example, benzoates) (increased risk of developing anaphylactic/anaphylactoid reactions).
– Severe impairment of liver and kidney function (the use of low doses is recommended due to the possibility of slowing the excretion of metamizole sodium).
If you have one of the listed diseases/conditions, be sure to consult your doctor before taking the drug.
Side Effects
Side Effects
Adverse reactions are classified as follows, according to the WHO (World Health Organization) classification: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1000 to <1/100), rare (≥1/10000 to <1/1000), very rare (up to <1/10000) and frequency unknown (cannot be estimated based on available data).
Heart disorders
Frequency unknown: Kounis syndrome (allergic coronary syndrome, manifested by clinical and laboratory signs of angina caused by inflammatory mediators).
Immune system disorders
Rarely: anaphylactic/anaphylactoid reactions.
Very rare: analgesic bronchial asthma.
Frequency unknown: anaphylactic shock.
Metamizole sodium may cause anaphylactic or anaphylactoid reactions, which in very rare cases can be severe and life-threatening. They can occur even if the drug has been taken many times before without any complications.
Such drug reactions may occur immediately or several hours after taking metamizole sodium, usually within one hour.
In milder cases, they manifest themselves in the form of skin and mucous membrane symptoms (itching, burning, flushing, urticaria, swelling) or in the form of shortness of breath or complaints from the gastrointestinal tract. In severe cases, these reactions progress to generalized urticaria, severe angioedema (especially involving the larynx), severe bronchospasm, cardiac arrhythmias, a sharp decrease in blood pressure (which is sometimes preceded by an increase in blood pressure) and the development of circulatory shock. In persons with analgesic bronchial asthma syndrome and intolerance to analgesic drugs, these reactions manifest themselves in the form of attacks of bronchial asthma.
Skin and subcutaneous tissue disorders
Uncommon: fixed drug dermatitis.
Rarely: skin rash.
Frequency unknown: Stevens-Johnson syndrome, Lyell’s syndrome (toxic epidermal necrolysis).
Disorders of the blood and lymphatic system
Rarely: leukopenia.
Very rare: agranulocytosis, including fatal cases and thrombocytopenia.
Frequency unknown: aplastic anemia, pancytopenia, including fatal cases.
These reactions are immunological reactions in nature. They can occur even if the drug has been previously taken many times without any complications.
Typical symptoms of agranulocytosis are lesions of the mucous membranes (horny cavity and pharynx, anorectal region and genital organs), sore throat, fever. However, when antibiotics are used, these phenomena may be mild. Sometimes, but not always, there is a slight enlargement of the lymph nodes or spleen.
The erythrocyte sedimentation rate increases significantly, the content of granulocytes is sharply reduced or they are not detected. As a rule, hemoglobin, red blood cells and platelets remain normal, but deviations may occur. Typical symptoms of thrombocytopenia are an increased tendency to bleed and the appearance of petechiae on the skin and mucous membranes.
If there is an unexpected deterioration in the general condition, the fever does not subside, or new or painful ulcerations appear on the mucous membranes, especially in the mouth, nose or throat, treatment tactics involve immediate discontinuation of the drug without waiting for laboratory results.
If pancytopenia develops, the drug should be discontinued and a complete blood count should be monitored until its values return to normal (see “Special Instructions”).
Vascular disorders
Uncommon: isolated arterial hypotension.
After taking the drug, an isolated transient decrease in blood pressure is possible (possibly pharmacologically caused and not accompanied by other manifestations of anaphylactic/anaphylactoid reactions); in rare cases, the decrease in blood pressure can be very pronounced. During fever, a dose-dependent sharp decrease in blood pressure without other signs of a hypersensitivity reaction is also possible.
Nocturnal and urinary tract disorders
Very rare: renal dysfunction.
Frequency unknown: interstitial nephritis.
In very rare cases, patients with impaired renal function may experience an acute deterioration in renal function (acute renal failure), in some cases with oliguria, anuria or proteinuria.
General disorders
Uncommon: urine may turn red due to the presence of a metabolite in the urine – rubazonic acid.
Interaction
Interaction
With cyclosporine
Metamizole sodium may cause a decrease in plasma concentrations of cyclosporine, therefore, when used simultaneously, the concentration of cyclosporine should be monitored.
With chlorpromazine
With the simultaneous use of metamizole sodium and chlorpromazine, severe hypothermia may develop.
With methotrexate
The simultaneous use of metamizole sodium and methotrexate or other myelotoxic drugs may increase the hematotoxicity of the latter, especially in elderly patients. Therefore, this combination should be avoided.
With other non-narcotic analgesics
The simultaneous use of metamizole sodium with other non-narcotic analgesics may lead to mutual enhancement of toxic effects.
With tricyclic antidepressants, oral contraceptives, allopurinol
Tricyclic antidepressants, oral contraceptives, allopurinol disrupt the metabolism of metamizole sodium in the liver and increase its toxicity.
With barbiturates, phenylbutazone and other inducers of microsomal liver enzymes
Barbiturates, phenylbutazone and other inducers of microsomal liver enzymes weaken the effect of metamizole sodium.
With sedatives and tranquilizers
Sedatives and tranquilizers enhance the analgesic effect of metamizole sodium.
With drugs that are highly bound to plasma proteins (oral hypoglycemic agents, indirect anticoagulants, glucocorticosteroids and indomethacin)
Metamizole sodium, displacing oral hypoglycemic agents, indirect anticoagulants, glucocorticosteroids and indomethacin from their association with plasma proteins, increases their activity.
With timazol
Timazol increases the risk of developing leukopenia.
With codeine, H2 blockers and propranolol
Codeine, H2-histamine receptor blockers and propranolol enhance the effects of metamizole sodium.
With acetylsalicylic acid (ASA)
With simultaneous use, metamizole sodium may reduce the effect of ASA on platelet aggregation. Therefore, this combination should be used with caution when treating patients taking ASA as an antiplatelet agent.
With bupropion
Metamizole sodium may reduce the concentration of bupropion in the blood, which should be taken into account when using them simultaneously.
With other medications
It is well known that pyrazolone derivatives can interact with indirect anticoagulants, captopril, lithium and triamterene, and also affect the effectiveness of antihypertensive drugs and diuretics. The drug interactions of metamizole sodium with these drugs have not yet been studied. Due to the increased risk of anaphylactic/anaphylactoid reactions, radiocontrast agents, colloidal blood substitutes and penicillin should not be used during treatment with metamizole sodium.
Overdose
Overdose
Do not exceed the recommended dose and duration of use!
Symptoms
Acute overdose is manifested by nausea, vomiting, abdominal pain, renal dysfunction/acute renal failure (for example, as a manifestation of interstitial nephritis) and, rarely, symptoms from the central nervous system (dizziness, drowsiness, coma, convulsions) and a decrease in blood pressure, leading to tachycardia and shock, heart rhythm disturbance (tachycardia), hypothermia, shortness of breath, acute agranulocytosis, hemorrhagic syndrome, paralysis of the respiratory muscles.
In high overdoses, excretion of rubazonic acid may turn the urine red.
Treatment
A specific antidote is not known. In case of a recent overdose, in order to limit the intake of the drug into the body, primary detoxification (for example, gastric lavage) or sorption therapy (for example, activated carbon) is carried out. The main metabolite (4N-methylaminoantipyrine) is removed by hemodialysis, hemofiltration, hemoperfusion and plasmafiltration.
Treatment of overdose, as well as prevention of serious complications, may require general and special intensive medical supervision and treatment.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 2 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 25 ° C. Store out of the reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | capsules |
| Brand | Pharmstandard-Leksredstva |
Other forms…
Related products
Buy Analgin ExtraCap, 500 mg capsules 10 pcs with delivery to USA, UK, Europe and over 120 other countries.