No products in the cart.
Anaferon, tablets 20 pcs
€12.33 €10.79
Description
In preventive and therapeutic use the drug has immunomodulatory and antiviral effect. Experimental and clinical effectiveness against influenza viruses and herpes simplex viruses of types 1 and 2 (herpes lip, genital herpes) is established.
The preparation decreases virus concentration in the affected tissues, affects the system of endogenous interferons and associated cytokines, induces formation of endogenous “early” interferons (IFN a/β) and gamma interferon (IFN γ).
Stimulates humoral and cellular immune response. Increases antibody production (including secretory IgA), activates Т-effectors and Т-helpers (Тх) functions, and normalizes their ratio. Increases the functional reserve of Tx and other cells involved in the immune response. It is an inducer of mixed Txl and Tx2-type immune response: it increases production of Txl cytokines (IFN γ, IL-2) and Tx2 (IL-4, 10), normalizes (modulates) the balance of Tx1/Tx2 activities. Increases the functional activity of phagocytes and natural killer cells (NK cells).
Indications
Indications
Anaferon® is indicated for use in adults.
Treatment and prevention of influenza and acute respiratory viral infections (ARVI) as part of complex therapy.
Complex treatment of newly diagnosed and recurrent herpes of the lips and genital herpes.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: immunostimulants; other immunostimulants.
ATX code
L03AX.
Pharmacological action
When used prophylactically and therapeutically, the drug has an immunomodulatory and antiviral effect. Experimental and clinical effectiveness has been established against influenza A and B viruses, parainfluenza, rhinovirus, respiratory syncytial virus, adenovirus, seasonal strains of coronaviruses, metapneumovirus, herpes simplex viruses types 1 and 2 (labial herpes, genital herpes). The drug reduces the concentration of the virus in the affected tissues, affects the system of endogenous interferons and associated cytokines, induces the formation of endogenous “early” interferons (IFN a/β) and interferon gamma (IFN γ).
Stimulates the humoral and cellular immune response. Increases the production of antibodies (including secretory IgA), activates the functions of
T-effectors, T-helpers (Tx), normalizes their ratio. Increases the functional reserve of Tx and other cells involved in the immune response. It is an inducer of a mixed Txl and Th2 type of immune response: it increases the production of Txl (IFN γ, IL-2) and Th2 (IL-4, 10) cytokines, normalizes (modulates) the balance of Th1/Th2 activities. Increases the functional activity of phagocytes and natural killer cells (NK cells).
Pharmacokinetics
The sensitivity of modern physicochemical methods of analysis (gas-liquid chromatography, high-performance liquid chromatography, gas chromatography-mass spectrometry) does not allow assessing the content of the active substance of the drug Anaferon® in biological fluids, organs and tissues, which makes it technically impossible to study pharmacokinetics.
Special instructions
Special instructions
The drug contains lactose monohydrate, and therefore is not recommended for use in patients with congenital galactosemia, glucose malabsorption syndrome, or congenital lactase deficiency.
Impact on the ability to drive vehicles and machinery
There are no data on the effect on the ability to drive vehicles and operate machinery.
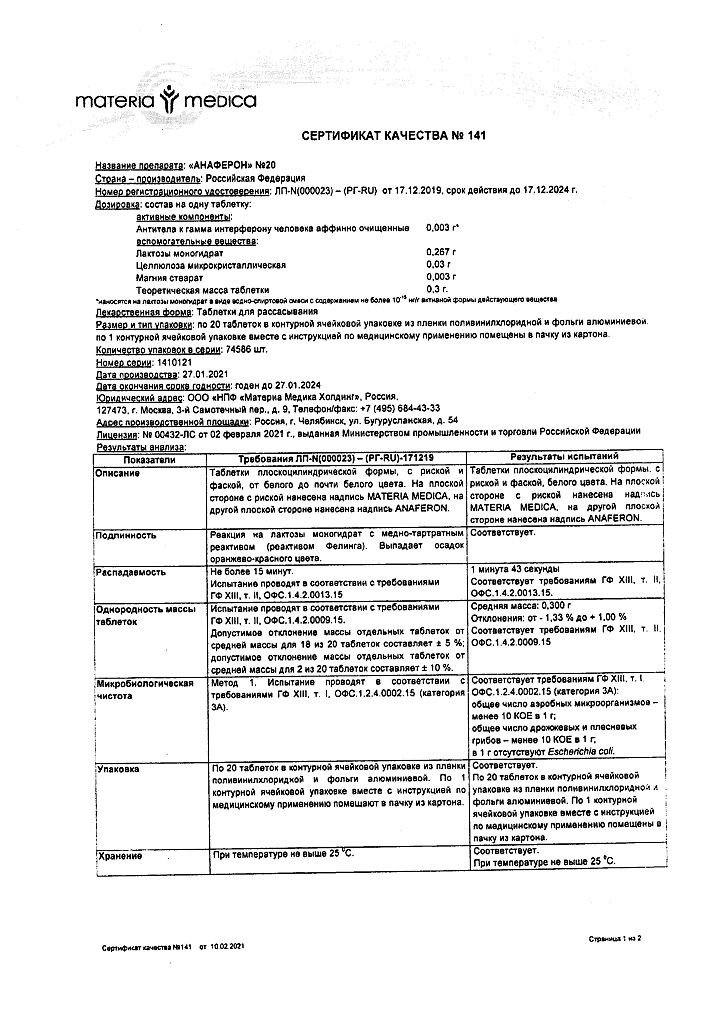
Composition
Composition
1 tablet contains:
Active ingredient:
Affinity purified antibodies to human interferon gamma – 10,000 EMD*.
Excipients: lactose monohydrate, microcrystalline cellulose, magnesium stearate.
* EMD – units of modifying action.
Pregnancy
Pregnancy
The safety of using Anaferon® in pregnant women and during lactation has not been studied. If it is necessary to take the drug, the benefit-risk ratio should be taken into account.
Contraindications
Contraindications
Increased individual sensitivity to the components of the drug.
Age up to 18 years.
Lactase deficiency, hereditary galactose intolerance, glucose-galactose malabsorption.
Side Effects
Side Effects
Allergic reactions and manifestations of increased individual sensitivity to the components of the drug are possible.
Interaction
Interaction
No cases of incompatibility with other drugs have been identified to date.
If necessary, the drug can be combined with other antiviral, antibacterial and symptomatic agents.
Overdose
Overdose
In case of overdose, dyspepsia may occur due to the excipients included in the drug.
Treatment: symptomatic.
Short product description
Short product description
Anaferon is a medicine against flu and colds.
The drug has a wide spectrum of antiviral activity, is characterized by a high safety profile, can be used for chronic diseases, and is compatible with any other drugs.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
During the period of use of the drug, store the blister pack in a cardboard box provided by the manufacturer.
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
Materia Medica Holding, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Materiala Medica Holding, Russia |
| Medication form | lozenges |
| Brand | Materiala Medica Holding |
Related products
Buy Anaferon, tablets 20 pcs with delivery to USA, UK, Europe and over 120 other countries.