No products in the cart.
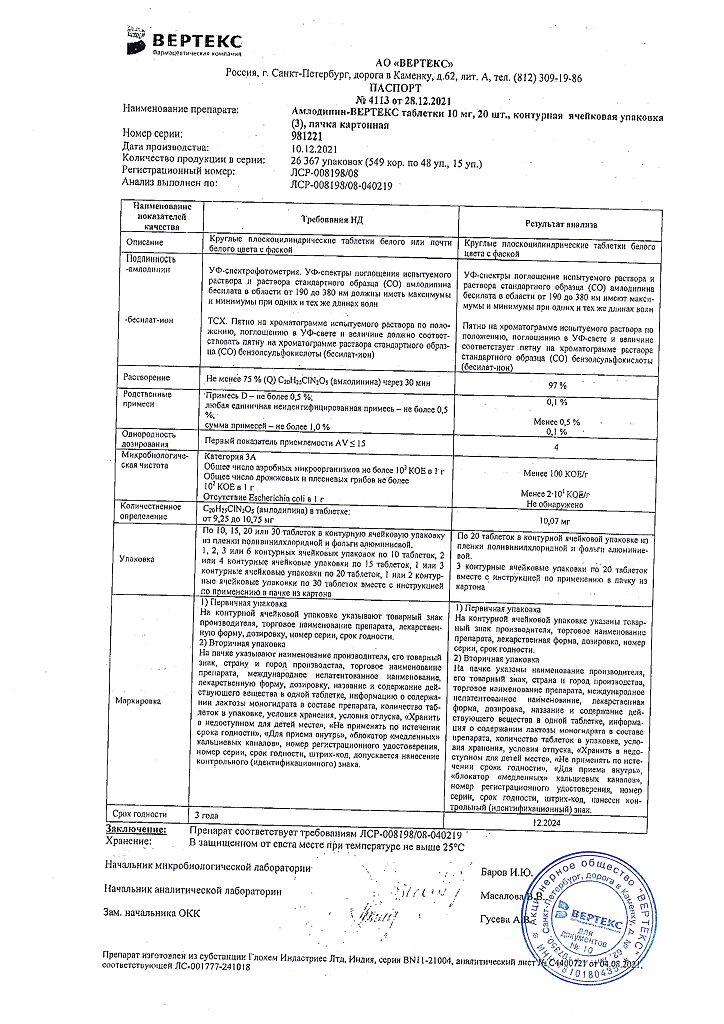
Amlodipine-Vertex, tablets 10 mg 60 pcs
€5.27 €4.68
Description
Pharmacodynamics
Amlodipine is a dihydropyridine derivative – blocker of “slow” calcium channels (BMCC) of II generation, has antianginal and hypotensive effect. By binding to dihydropyridine receptors, it blocks calcium channels, reduces transmembrane transfer of calcium ions into cell (more in vascular smooth muscle cells than in cardiomyocytes).
The antianginal action is due to the dilation of coronary and peripheral arteries and arterioles: in angina pectoris it reduces the severity of myocardial ischemia; by dilation of peripheral arterioles it reduces total peripheral vascular resistance; reduces the preload on the heart, myocardial oxygen demand. Dilates the main coronary arteries and arterioles in unchanged and ischemic areas of the myocardium, increases the flow of oxygen to the myocardium (especially in vasospastic angina); prevents the development of coronary artery spasm (including that caused by smoking). In patients with angina a single daily dose increases exercise tolerance, delays the development of another angina attack and “coronary” ST-segment depression; reduces the frequency of angina attacks and nitroglycerin consumption.
Amlodipine has a long-term dose-dependent hypotensive effect, which is due to direct vasodilator effect on vascular smooth muscle. In arterial hypertension a single daily dose of amlodipine provides clinically significant reduction of arterial pressure (BP) for 24 hours (in “lying” and “standing” position of the patient).
Limits the degree of left ventricular myocardial hypertrophy, has anti-atherosclerotic and cardioprotective effects in coronary heart disease (CHD). It does not affect myocardial contractility and conduction, inhibits platelet aggregation, increases glomerular filtration rate, has a weak natriuretic effect. In diabetic nephropathy it does not increase the severity of microalbuminuria. It has no adverse effect on metabolism and blood plasma lipid concentration. Time of onset of therapeutic effect is 2-4 hours, duration – 24 hours.
Pharmacokinetics
After oral administration amlodipine is slowly absorbed from the gastrointestinal tract. Food intake has no effect on the absorption of amlodipine. The average absolute bioavailability is 64%. Maximum serum concentration is observed after 6-9 hours. The equilibrium concentration is reached after 7-8 days of therapy. The binding to plasma proteins is 95%. Average volume of distribution is 21 l/kg body weight. Amlodipine undergoes slow but active metabolism (90 to 97%) in the liver with no significant “first pass” effect. Metabolites have no significant pharmacological activity.
The elimination half-life (T1/2) averages 35 hours. T1/2 in patients with arterial hypertension is 48 hours, in elderly patients it is increased up to 65 hours, in hepatic insufficiency – up to 60 hours, similar parameters of increase of T1/2 are observed in severe chronic heart failure, in renal function disorders – it does not change.
About 60% of a dose taken by mouth is excreted by kidneys mainly as metabolites, 10% – as unchanged, 20-25% – in bile and intestine as metabolites, as well as with breast milk.
The total clearance of amlodipine is 0.116 ml/s/kg (7ml/min/kg, 0.42 l/h/kg).
Amlodipine penetrates the blood-brain barrier. It is not excreted by hemodialysis.
Indications
Indications
Arterial hypertension (monotherapy or in combination with other antihypertensive drugs);
Stable angina pectoris and Prinzmetal angina (monotherapy or in combination with other antianginal drugs).
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Amlodipine, a dihydropyridine derivative, is a second-generation slow calcium channel blocker (SCCC) that has antianginal and hypotensive effects. By binding to dihydropyridine receptors, it blocks calcium channels and reduces the transmembrane transition of ions. calcium into the cell (more into vascular smooth muscle cells than into cardiomyocytes).
The antianginal effect is due to the expansion of coronary and peripheral arteries and arterioles: in case of angina pectoris, it reduces the severity of myocardial ischemia; by expanding peripheral arterioles, reduces total peripheral vascular resistance; reduces preload on the heart and myocardial oxygen demand. Expands the main coronary arteries and arterioles in unchanged and ischemic areas of the myocardium, increases the supply of oxygen to the myocardium (especially with vasospastic angina); prevents the development of spasm of the coronary arteries (including those caused by smoking). In patients with angina, a single daily dose increases tolerance to physical activity, delays the development of the next attack of angina and “ischemic” depression of the ST segment; reduces the frequency of angina attacks and nitroglycerin consumption.
Amlodipine has a long-term dose-dependent hypotensive effect, which is due to a direct vasodilating effect on vascular smooth muscle. For arterial hypertension, a single daily dose of amlodipine provides a clinically significant decrease in blood pressure (BP) over 24 hours (in the patient’s “lying” and “standing” position).
Reduces the degree of left ventricular myocardial hypertrophy, has an antiatherosclerotic and cardioprotective effect in coronary heart disease (CHD). It has no effect on myocardial contractility and conductivity, inhibits platelet aggregation, increases glomerular filtration rate, and has a weak natriuretic effect. In diabetic nephropathy, it does not increase the severity of microalbuminuria. Does not have an adverse effect on metabolism and plasma lipid concentrations. The onset of the therapeutic effect is 2–4 hours, duration is 24 hours.
Pharmacokinetics
After oral administration, amlodipine is slowly absorbed from the gastrointestinal tract. Food intake does not affect the absorption of amlodipine. The average absolute bioavailability is 64%. The maximum concentration in the blood serum is observed after 6–9 hours. Equilibrium concentration is achieved after 7–8 days of therapy. Communication with blood plasma proteins is 95%. The average volume of distribution is 21 l/kg body weight. Amlodipine undergoes slow but active metabolism (90-97%) in the liver with no significant first-pass effect. Metabolites do not have significant pharmacological activity.
The half-life (T1/2) averages 35 hours. T1/2 in patients with arterial hypertension is 48 hours, in elderly patients it increases to 65 hours, in case of liver failure – up to 60 hours, similar parameters for increasing T1/2 are observed in severe chronic heart failure, and in case of impaired renal function it does not change.
About 60% of the dose taken orally is excreted by the kidneys mainly in the form of metabolites, 10% unchanged, 20–25% with bile and through the intestines in the form of metabolites, as well as with breast milk.
The total clearance of amlodipine is 0.116 ml/s/kg (7 ml/min/kg, 0.42 l/h/kg).
Amlodipine penetrates the blood-brain barrier. It is not excreted during hemodialysis.
Special instructions
Special instructions
During treatment with Amlodipine, it is necessary to monitor patients’ body weight and the amount of sodium salt they consume; an appropriate low-salt diet is prescribed.
It is necessary to maintain dental hygiene and regularly visit the dentist (to prevent pain, bleeding and gum hyperplasia).
The dosage regimen of Amlodipine in elderly patients is similar to that in patients of other age groups. When increasing the dose, careful monitoring of elderly patients is necessary.
Despite the absence of withdrawal syndrome in BMCC, a gradual dose reduction is recommended before stopping treatment.
Amlodipine does not affect plasma concentrations in the blood of potassium ions, glucose, triglycerides, total cholesterol, low-density lipoproteins, uric acid, creatinine and urea nitrogen.
Abrupt discontinuation of the drug should be avoided due to the risk of worsening angina.
Amlodipine tablets are not recommended for hypertensive crisis.
Patients with low body weight, patients of short stature and patients with severe liver dysfunction may require a lower dose.
Impact on the ability to drive vehicles and operate machinery
There have been no reports of the effect of Amlodipine on driving or operating machinery. However, some patients, mainly at the beginning of treatment, may experience drowsiness and dizziness. If they occur, care should be taken when driving vehicles and engaging in potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Amlodipine
Composition
Composition
1 tablet contains
active ingredient:
amlodipine besylate in terms of amlodipine 10 mg;
excipients:
microcrystalline cellulose,
lactose (milk sugar),
croscarmellose sodium (primellose),
calcium stearate (calcium octadecanoate).
Pregnancy
Pregnancy
Amlodipine was not found to be teratogenic in animal studies, but there is no clinical experience with its use during pregnancy and lactation.
Therefore, amlodipine should not be prescribed to pregnant women and during lactation, as well as to women of childbearing age if they do not use reliable methods of contraception.
Contraindications
Contraindications
hypersensitivity to amlodipine, other dihydropyridine derivatives, and other components of the drug;
severe arterial hypotension (systolic blood pressure less than 90 mm Hg);
collapse;
cardiogenic shock;
unstable angina (with the exception of Prinzmetal’s angina);
severe aortic stenosis;
age under 18 years (efficacy and safety have not been established);
lactose intolerance, lactase deficiency or glucose-galactose malabsorption;
pregnancy;
lactation period.
With caution: impaired liver function, sick sinus syndrome (severe bradycardia, tachycardia), chronic heart failure of non-ischemic etiology of functional class III-IV according to the NYHA classification, aortic stenosis, mitral stenosis, hypertrophic obstructive cardiomyopathy, acute myocardial infarction (and within 1 month after), old age.
Side Effects
Side Effects
From the cardiovascular system: often – palpitations, peripheral edema (swelling of the ankles and feet), infrequently – excessive decrease in blood pressure, orthostatic hypotension, vasculitis; rarely – development or worsening of chronic heart failure; very rarely – rhythm disturbances (bradycardia, ventricular tachycardia, atrial fibrillation), myocardial infarction, chest pain, migraine.
From the central nervous system: often – headache, dizziness, increased fatigue; uncommon – malaise, fainting, asthenia, hypoesthesia, paresthesia, peripheral neuropathy, tremor, insomnia, emotional lability, unusual dreams, nervousness, depression, anxiety; rarely – convulsions, apathy, agitation; very rarely – ataxia, amnesia.
From the hematopoietic organs: very rarely – thrombocytopenia, leukopenia, thrombocytopenic purpura.
From the respiratory system: infrequently – shortness of breath, rhinitis; very rarely – cough.
From the digestive tract: often – nausea, abdominal pain; uncommon – vomiting, change in bowel habits (including constipation, flatulence), dyspepsia, diarrhea, anorexia, dry oral mucosa, thirst; rarely – gum hyperplasia, increased appetite; very rarely – gastritis, pancreatitis, hyperbilirubinemia, jaundice (usually cholestatic), increased activity of liver transaminases, hepatitis.
From the genitourinary system: infrequently – pollakiuria, painful urge to urinate, nocturia, impotence; very rarely – dysuria, polyuria.
From the skin: rarely – increased sweating, very rarely – cold sticky sweat, xeroderma, alopecia, dermatitis, purpura, change in skin color.
Allergic reactions: infrequently – skin itching, rash; very rarely – angioedema, erythema multiforme, urticaria.
From the musculoskeletal system: infrequently – arthralgia, muscle cramps, arthrosis, myalgia (with long-term use), back pain; rarely – myasthenia.
Other: infrequently – alopecia, tinnitus, gynecomastia, weight gain/loss, blurred vision, diplopia, accommodation disturbance, xerophthalmia, conjunctivitis, eye pain, taste perversion, chills, nosebleeds, increased sweating; rarely – dermatitis; very rarely – cold sticky sweat, parosmia, skin pigmentation disorder, hyperglycemia.
Interaction
Interaction
Inhibitors of microsomal oxidation may increase the concentration of amlodipine in the blood plasma, increasing the risk of side effects, and inducers of microsomal liver enzymes may reduce this indicator.
Unlike other BMCCs, amlodipine does not have clinically significant interactions with non-steroidal anti-inflammatory drugs, especially indomethacin.
Thiazide and loop diuretics, beta-blockers, verapamil, ACE inhibitors and nitrates enhance the antianginal or hypotensive effects of amlodipine.
Amiodarone, quinidine, alpha1-blockers, antipsychotics (neuroleptics) and isoflurane may enhance the hypotensive effect of amlodipine.
Calcium supplements may reduce the effect of BMCC.
When amlodipine is used together with lithium drugs, it is possible to increase the manifestations of neurotoxicity of the latter (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
Amlodipine has no effect on the pharmacokinetic parameters of digoxin and warfarin. Cimetidine does not affect the pharmacokinetics of amlodipine.
Antiviral drugs (ritonavir) help increase the concentrations of BMCC (including amlodipine) in the blood plasma.
Overdose
Overdose
Symptoms:
a pronounced decrease in blood pressure with the possible development of reflex tachycardia and excessive peripheral vasodilation (risk of developing severe and persistent arterial hypotension, including the development of shock and death).
Treatment:
gastric lavage, administration of activated charcoal (especially in the first 2 hours after an overdose), maintaining the function of the cardiovascular system, monitoring indicators of heart and lung function, Trendelenburg position, monitoring circulating blood volume and diuresis. To restore vascular tone, use vasoconstrictor drugs (in the absence of contraindications to their use); to eliminate the effects of blockade of calcium channels – intravenous administration of calcium gluconate. Hemodialysis is not effective.
Storage conditions
Storage conditions
Store in a dry place, protected from light, at a temperature not exceeding 25°C.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Store in a dry, dark place at a temperature not exceeding 25oC. |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Amlodipine-Vertex, tablets 10 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.