No products in the cart.
Amlodipine, 10 mg tablets, 20 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group: slow calcium channel blocker.
The ATC code: C08CA01
Pharmacological properties
Pharmacodynamics
Slow calcium channel blocker (SCB), dihydropyridine derivative, has antianginal and antihypertensive effect. It blocks calcium channels, reduces transmembrane transition of calcium ions into the cell (more in vascular smooth muscle cells than in cardiomyocytes).
The antianginal action is due to the dilation of coronary and peripheral arteries and arterioles:
In angina, it reduces the severity of myocardial ischemia; by dilating the peripheral arterioles, it reduces total peripheral vascular resistance, reduces the afterload on the heart, and reduces myocardial oxygen demand;
– expanding coronary arteries and arterioles in unchanged and in ischemic areas of the myocardium, increases the flow of oxygen to the myocardium (especially in vasospastic angina); prevents spasm of coronary arteries (including those caused by smoking).Also caused by smoking).
In patients with stable angina a single daily dose increases exercise tolerance, slows down the development of angina and “coronary” ST-segment depression, reduces the frequency of angina attacks and the consumption of nitroglycerin and other nitrates.
It has a long-term dose-dependent antihypertensive effect. The antihypertensive effect is due to a direct vasodilatory effect on vascular smooth muscle. In arterial hypertension, a single dose provides clinically significant reduction of blood pressure (BP) for 24 hours (in patient’s “lying” and “standing” position). Orthostatic hypotension with amlodipine is rare. It does not cause a decrease in exercise tolerance and left ventricular ejection fraction. It reduces the degree of left ventricular myocardial hypertrophy. It does not affect myocardial contractility and conduction, does not cause reflex increase in heart rate (HR), inhibits platelet aggregation, increases glomerular filtration rate, has a weak natriuretic effect. In diabetic nephropathy it does not increase the severity of microalbuminuria.
It does not have any adverse effect on metabolism and plasma lipid concentration and can be used for treatment of patients with bronchial asthma, diabetes and gout. Significant decrease in BP is observed after 6-10 hours, the duration of effect is 24 hours.
. In patients with diseases of the cardiovascular system, including coronary atherosclerosis with lesions of one vessel and up to stenosis of 3 or more arteries, atherosclerosis of the carotid arteries, who had myocardial infarction, percutaneous transluminal angioplasty (TLAP) of coronary arteries or patients with angina pectoris, use of amlodipine prevents the development of carotid intima-media thickening, reduces mortality from myocardial infarction, stroke, TLAP, aorto-coronary bypass surgery; leads to lower incidence of unstable angina and progression of chronic heart failure (CHF); reduces the frequency of interventions to restore coronary blood flow.
Does not increase risk of death or complications and lethal outcomes in patients with CHF (functional class III-IV according to NYHA classification) during therapy with digoxin, diuretics and angiotensin-converting enzyme inhibitors (ACE). In patients with CHF (functional class III-IV according to NYHA classification) of non-ischemic etiology when using amlodipine there is a possibility of pulmonary edema.
Pharmacokinetics
After oral administration, amlodipine is slowly absorbed from the gastrointestinal tract. Mean absolute bioavailability is 64-80%, maximum serum concentrations are observed after 6-12 hours. Equilibrium concentrations are reached after 7-8 days of therapy. Simultaneous intake of food has no effect on absorption of amlodipine. The average volume of distribution is 21 l/kg body weight, indicating that most of the drug is in the tissues and a relatively smaller part is in the blood. Most of the drug that is in the blood (97.5%) is bound to plasma proteins.
Amlodipine undergoes slow but active metabolism (90%) in the liver to form inactive metabolites, with no significant effect of “primary passage” through the liver. Metabolites have no significant pharmacological activity.
After a single oral administration, the elimination half-life (T1/2) varies from 31 to 50 hours, with a repeated use T1/2 of approximately 45 hours. About 60% of oral intake is excreted by kidneys mainly as metabolites, 10% – unchanged, and 20-25% via intestine with bile. Total clearance of amlodipine is 0.116 ml/s/kg (7 ml/min/kg, 0.42 l/h/kg).
In elderly patients (over 65 years) the excretion of amlodipine is slower (T1/2 – 65 h) compared to younger patients, but this difference has no clinical significance.
In patients with hepatic impairment, a prolonged T1/2 is expected and cumulation of the drug in the body will be higher with long-term use (T1/2 up to 60 h).
Renal insufficiency has no significant effect on the kinetics of amlodipine.
Amlodipine penetrates the blood-brain barrier. It is not eliminated by hemodialysis.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Overly, once daily with the required amount of water (100 ml).
In case of arterial hypertension and angina pectoris, the initial dose is 5 mg once daily. If there is no therapeutic effect within 2-4 weeks, the dose of the drug may be increased to a maximum daily dose of 10 mg.
In elderly patients
Amlodipine is recommended in an average therapeutic dose; no dose adjustment is required.
In patients with hepatic impairment
Although the T1/2 of amlodipine, like all BMKs, is prolonged, in patients with hepatic impairment, no dose adjustment is usually required (see section “Special Precautions”).
In patients with renal impairment
Amlodipine is recommended in usual doses, but a possible slight increase in T1/2 must be considered (see section “Special Precautions”).
There is no need to change the dose when concomitantly prescribed with thiazide diuretics, beta-adrenoblockers and angiotensin-converting enzyme inhibitors (ACE).
Interaction
Interaction
Amlodipine may be safely used for therapy of arterial hypertension together with thiazide diuretics, alpha-adrenoblockers and beta-adrenoblockers or angiotensin-converting enzyme inhibitors (ACE).
In patients with stable angina pectoris, amlodipine may be used with other antianginal agents, e.g., long- or short-acting nitrates, beta-adrenoblockers.
Unlike other DMICs, no clinically significant interactions have been found with amlodipine when co-administered with nonsteroidal anti-inflammatory drugs (NSAIDs), including indomethacin.
The antianginal and antihypertensive effect of BMKK may be enhanced when used with thiazide and loop diuretics, ACE inhibitors with and nitrates, beta-adrenoblockers, and increased antihypertensive effect when combined with alpha 1-adrenoblockers, neuroleptics.
Beta-adrenoblockers when used concomitantly with amlodipine may aggravate chronic heart failure.
Amlodipine may be safely used with oral antibiotics and hypoglycemic agents.
While no negative inotropic effects have generally been observed with amlodipine in studies, however, some PBMCs can exacerbate the negative inotropic effects of antiarrhythmic agents that cause QT interval prolongation (e.g., amiodarone and quinidine).
The concomitant use of simvastatin at a dose of 80 mg daily and amlodipine at a dose of 10 mg daily leads to a 77% increase in simvastatin exposure. It is recommended to reduce the dose of simvastatin in patients taking amlodipine to 20 mg daily.
A single administration of 100 mg sildenafil in patients with arterial hypertension has no effect on amlodipine pharmacokinetic parameters.
Concomitant use with sildenafil requires BP control (risk of arterial hypotension).
The repeated use of amlodipine 10 mg and atorvastatin 80 mg is not associated with significant changes in pharmacokinetics of atorvastatin.
Ethanol (beverages containing alcohol): amlodipine at a single and repeated use in a dose of 10 mg has no effect on the pharmacokinetics of ethanol.
Antiviral agents (ritonavir) increase plasma concentrations of PBMCs, including amlodipine.
Neuroleptics and isoflurane increase the antihypertensive effect of dihydropyridine derivatives.
Calcium preparations may decrease the effect of BMCCs.
When concomitant use of PBMCs with lithium preparations (no data for amlodipine), there may be an increase in their neurotoxicity (nausea, vomiting, diarrhea, ataxia, tremor, tinnitus).
There have been no studies of concomitant use of amlodipine and cyclosporine in healthy volunteers and all groups of patients, except for patients after kidney transplantation. Various studies of amlodipine with cyclosporine in patients after kidney transplantation show that the use of this combination may not lead to any effect, or increase the minimum concentration of cyclosporine to varying degrees up to 40%. These data should be taken into account and cyclosporine concentrations should be monitored in this group of patients when concomitant use of cyclosporine and amlodipine.
Concomitant use of amlodipine may increase systemic plasma exposure of tasonermine. In such cases, regular monitoring of tasonermine in blood and dose adjustment if necessary are required.
The serum concentrations of digoxin and its renal clearance are not affected.
There is no significant effect on the effect of warfarin (prothrombin time).
Cimetidine does not affect the pharmacokinetics of amlodipine.
In in vitro studies amlodipine does not affect the binding to plasma proteins of digoxin, phenytoin, warfarin and indomethacin.
Grapefruit juice: Concomitant administration of 240 mg of grapefruit juice and 10 mg of oral amlodipine is not accompanied by significant changes in pharmacokinetics of amlodipine. However, it is not recommended to use grapefruit juice and amlodipine concomitantly, since a genetic polymorphism of CYP3A4 isoenzyme may increase bioavailability of amlodipine and, consequently, increase antihypertensive effect.
Aluminum- or magnesium-containing antacids: their single administration has no significant effect on the pharmacokinetics of amlodipine.
CYP3A4 isoenzyme inhibitors: Concomitant use of diltiazem 180 mg and amlodipine 5 mg in patients aged 69 to 87 years with arterial hypertension showed 57% increase of systemic amlodipine exposure. Concomitant use of amlodipine and erythromycin in healthy volunteers (18 to 43 years) does not result in significant changes in amlodipine exposure (22% increase in area under the curve “concentration-time” (AUC)). Although the clinical significance of these effects is unclear, they may be more pronounced in older patients.
Powerful inhibitors of CYP3A4 isoenzyme (e.g., ketoconazole, itraconazole) may lead to increased plasma concentrations of amlodipine to a greater extent than diltiazem. Caution should be exercised when using amlodipine and CYP3A4 isoenzyme inhibitors.
Clarithromycin: CYP3A4 isoenzyme inhibitor. Patients taking clarithromycin and amlodipine concomitantly have an increased risk of BP decrease. Patients taking this combination are advised to be under close medical supervision.
CYP3A4 isoenzyme inducers: There are no data on the effect of CYP3A4 isoenzyme inducers on amlodipine pharmacokinetics. BP should be carefully monitored when concomitant use of amlodipine and CYP3A4 isoenzyme inducers.
Tacrolimus: when used concomitantly with amlodipine there is a risk of increased plasma concentrations of tacrolimus. To avoid tacrolimus toxicity when used concomitantly with amlodipine, plasma concentrations of tacrolimus should be monitored and the dose of tacrolimus should be adjusted if necessary.
Special Instructions
Special Instructions
The efficacy and safety of amlodipine in hypertensive crisis have not been established.
Amlodipine therapy requires control of body weight and table salt intake, an appropriate diet is indicated.
Maintain oral hygiene and see a dentist (to prevent soreness, bleeding, and gum hyperplasia).
When using amlodipine in patients with chronic heart failure of NYHA functional class III and IV nonischemic genesis, an increased incidence of pulmonary edema has been noted, despite no signs of worsening of the course of chronic heart failure.
In acute myocardial infarction amlodipine is used after hemodynamic stabilization (see section “Contraindications”).
Patients with hepatic impairment should be under medical supervision if Amlodipine is required.
In elderly patients the T1/2 may increase and the drug clearance may decrease. No change in dosage is required, but closer monitoring of patients in this category is necessary.
Patients of low body weight, short patients and patients with significant hepatic impairment may require lower doses of the drug.
Patients with impaired renal function require monitoring.
While slow calcium channel blockers have no withdrawal syndrome, a gradual reduction in doses is recommended before discontinuing treatment.
Impact on the ability to drive motor transport and other complex mechanisms
. Although no adverse effect on the ability to drive or operate motor transport or other complex machines has been observed while taking Amlodipine, however due to possible excessive decrease of BP, dizziness, somnolence and other adverse reactions, caution should be exercised in the listed situations, especially at the beginning of treatment and when increasing the dose.
Contraindications
Contraindications
Side effects
Side effects
According to the World Health Organization, adverse effects are classified according to their frequency of development as follows: very common (â¥10% of appointments); common (â¥1% and < 10%); infrequent (â¥0.1% and < 1%); rare (â¥0.01% and < 0.1%); very rare (< 0.01%); frequency unknown (insufficient data to estimate frequency of development).
Central nervous system disorders: frequent – headache, dizziness, increased fatigue, somnolence; infrequent – general malaise, asthenia, hypoesthesia, paresthesia, peripheral neuropathy, tremor, insomnia, mood lability, unusual dreams, hyperexcitability, depression, anxiety, perversion of taste; very rare – migraine, apathy, agitation, ataxia, amnesia, asthenia, increased sweating; frequency unknown – extrapyramidal disorders.
Digestive system disorders; frequently – nausea, abdominal pain; infrequently – vomiting, constipation or diarrhea, flatulence, dyspepsia, anorexia, dry oral mucosa, thirst; rarely – hyperplasia of gums, increased appetite; very rarely – pancreatitis, gastritis, jaundice (due to cholestasis), hyperbilirubinemia, increased activity of “liver” transaminases, hepatitis.
Cardiovascular system disorders: frequent – palpitations, “rushes” of blood to the skin of the face, peripheral edema (ankles and feet); infrequent – excessive reduction of BP; very rare – fainting, shortness of breath, vasculitis, orthostatic hypotension, development or aggravation of the course of CHF, heart rhythm disorders (including bradycardia, ventricular tachycardia and atrial fibrillation), myocardial infarction, pain in the chest, pulmonary edema.
Hematopoietic and lymphatic system disorders: very rarely – thrombocytopenic purpura, leukopenia, thrombocytopenia.
Urinary system disorders: infrequent – frequent urination, painful urination, nycturia; very rare – dysuria, polyuria.
With the reproductive system and mammary glands: infrequent – gynecomastia, erectile dysfunction.
Respiratory system disorders: infrequent dyspnea, rhinitis, nasal bleeding; very rare – cough.
Muscular system: infrequent – muscle cramps, myalgia, arthralgia, back pain, arthrosis; rarely – myasthenia.
The skin: rarely – dermatitis; very rarely – alopecia, xeroderma, cold sweats, impaired skin pigmentation.
Allergic reactions: infrequent – skin itching, skin rash (including erythematous, maculopapular rash, urticaria); very rare – angioedema, erythema multiforme.
Senses: infrequent – tinnitus, diplopia, accommodation disorder, xerophthalmia, conjunctivitis, eye pain, visual disturbances; very rare – parosmia.
Metabolism disorders: very rarely hyperglycemia; infrequently weight gain/loss.
Others: infrequent – chills, pain of unspecified localization.
Overdose
Overdose
Similarities
Similarities
Additional information
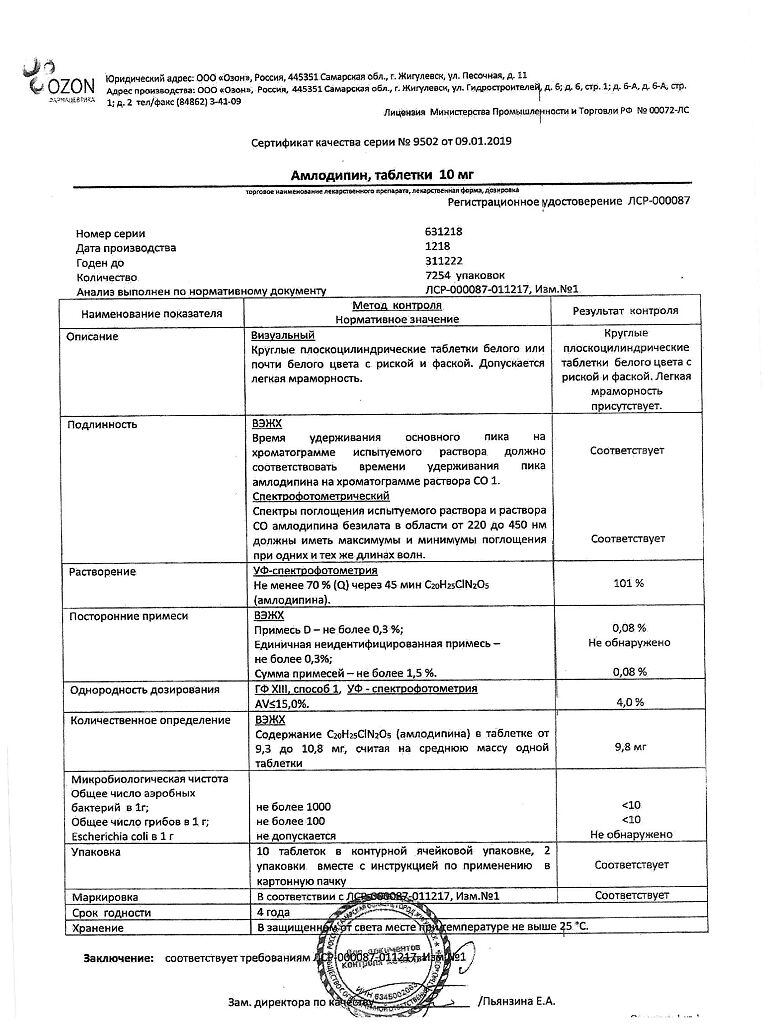
| Shelf life | 4 years. |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Ozon, Russia |
| Medication form | pills |
| Brand | Ozon |
Other forms…
Related products
Buy Amlodipine, 10 mg tablets, 20 pcs. with delivery to USA, UK, Europe and over 120 other countries.