No products in the cart.
Almont, 5 mg 28 pcs.
€33.16 €27.63
Description
Bronchospasm, Runny nose (rhinitis), Bronchial asthma
Prevention and long-term treatment of bronchial asthma in children, including:
- preventing daytime and nighttime symptoms of the disease (for children 2 years and older);
- Treatment of bronchial asthma in patients with hypersensitivity to acetylsalicylic acid (for children 6 years and older);
- preventing exercise-induced bronchospasm (for children 2 years and older).
Promotion of symptoms of seasonal and year-round allergic rhinitis in children from 2 years of age.
Indications
Indications
Prevention and long-term treatment of bronchial asthma in children, including:
prevention of daytime and nighttime symptoms of the disease (for children aged 2 years and older);
treatment of bronchial asthma in patients with hypersensitivity to acetylsalicylic acid (for children aged 6 years and older);
prevention of bronchospasm caused by physical activity (for children aged 2 years and older).
Relief of symptoms of seasonal and year-round allergic rhinitis in children from 2 years of age.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group:
Special instructions
Special instructions
It is NOT recommended to prescribe for the treatment of acute attacks of bronchial asthma. Patients with bronchial asthma are advised to always carry emergency medications with them. When an acute attack occurs, short-acting inhaled beta2-agonists should be used. Patients should consult their physician as soon as possible if they require more inhaled short-acting beta2-agonists than usual.
Do not suddenly replace the drug with inhaled or oral corticosteroids. There is no data proving the possibility of reducing the dose of oral corticosteroids during concomitant use.
In rare cases, patients receiving antiasthmatic drugs, including montelukast, may develop systemic eosinophilia, sometimes accompanied by clinical signs of vasculitis, the so-called Charge-Strauss syndrome, a condition that is treated with systemic corticosteroids. These cases are usually associated with dose reduction or discontinuation of oral corticosteroid therapy. The possibility that leukotriene receptor antagonists may be associated with the development of Charge-Strauss syndrome cannot be excluded or established. Therefore, physicians should be alerted to the possibility of eosinophilia, vascular rash, increased severity of pulmonary symptoms, cardiac complications, and/or neuropathy in patients. Patients who develop the above symptoms should be re-evaluated and their treatment regimen reviewed. Treatment with the drug does not prevent the development of bronchospasm in patients with hypersensitivity to acetylsalicylic acid when using acetylsalicylic acid and other NSAIDs.
Montelast contains aspartame, a source of phenylalanine. This drug may cause harm to the health of patients with phenylketonuria.
The drug contains lactose monohydrate and should not be taken by patients with rare hereditary diseases: galactose intolerance, lactase deficiency or glucose-galactose malabsorption.
Impact on the ability to drive vehicles and operate machinery
As a rule, montelukast does not affect the ability to drive vehicles or operate other mechanisms, but very rarely, some patients have experienced drowsiness and dizziness; when these signs appear, patients are not recommended to drive vehicles or engage in other activities that require concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Montelukast
Composition
Composition
Active substance:
montelukast 5.00 mg (as montelukast sodium 5.20 mg);
excipients:
mannitol 201.20 mg,
microcrystalline cellulose 66.00 mg,
hyprolose 9.00 mg,
croscarmellose sodium 9.00 mg,
dye Pigment Blend PB-24880 (lactose monohydrate 4.50 mg, red iron oxide dye 0.50 mg) 5.00 mg
magnesium stearate 3.00 mg,
aspartame 1.50 mg,
cherry flavor (Silarom Cherry Flavor 1219813182) 0.10 mg.
Contraindications
Contraindications
Hypersensitivity to the active or any excipient of the drug;
children up to 2 years of age (for a dosage of 4 mg) and up to 6 years of age (for a dosage of 5 mg);
patients with rare hereditary diseases: galactose intolerance, lactase deficiency or glucose-galactose malabsorption;
phenylketonuria (contains aspartame).
Side Effects
Side Effects
Infectious and parasitic diseases: upper respiratory tract infections.
From the blood and lymphatic system: increased tendency to bleeding, thrombocytopenia.
From the immune system: hypersensitivity reactions, incl. anaphylaxis, eosinophilic infiltration of the liver.
From the psyche: pathological dreams (including nightmares), hallucinations, insomnia, somnambulism, irritability, anxiety, restlessness, agitation (including aggressive behavior or hostility), tremor, depression, disorientation, suicidal thoughts and behavior (suicidality).
From the nervous system: headache, dizziness, drowsiness, paresthesia/hypoesthesia, convulsions.
From the cardiovascular system: palpitations.
From the respiratory system: nosebleeds.
From the digestive system: diarrhea, dry mouth, dyspepsia, nausea, vomiting, abdominal pain, pancreatitis.
From the liver and biliary tract: increased activity of ALT and AST, hepatitis (including cholestatic, hepatocellular and mixed liver lesions).
From the skin and subcutaneous tissues: angioedema, tendency to hematomas, urticaria, itching, rash, erythema nodosum, erythema multiforme.
Musculoskeletal and connective tissue disorders: arthralgia, myalgia, including muscle cramps.
General disorders: asthenia/fatigue, malaise, edema, pyrexia, thirst.
Other: In very rare cases, the development of Charge-Strauss syndrome has been reported during treatment with montelukast.
Interaction
Interaction
In patients simultaneously receiving phenobarbital, the area under the pharmacokinetic concentration-time curve of montelukast decreased by approximately 40%, but no dosage adjustment is required in such patients. Since montelukast is metabolized by CYP3A4, caution should be exercised, especially in children, if montelukast is co-administered with CYP3A4 inducers such as phenytoin, phenobarbital and rifampicin.
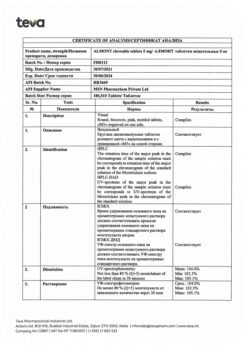
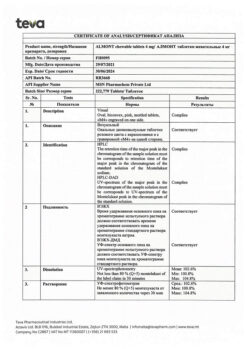
Specifications
Specifications
Prevention and long-term treatment of bronchial asthma, including prevention of symptoms of the disease during the day and night, treatment of bronchial asthma in patients with hypersensitivity to acetylsalicylic acid, prevention of bronchospasm caused by physical activity.
Relief of symptoms of seasonal and persistent allergic rhinitis.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25°C.
Keep out of the reach of children!
Manufacturer
Manufacturer
Iceland
Additional information
| Conditions of storage | Store in a dry, light-protected place at a temperature not exceeding 25°C. Keep out of reach of children! |
|---|---|
| Manufacturer | Iceland |
| Medication form | chewable tablets |
Other forms…
Related products
Buy Almont, 5 mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.