No products in the cart.
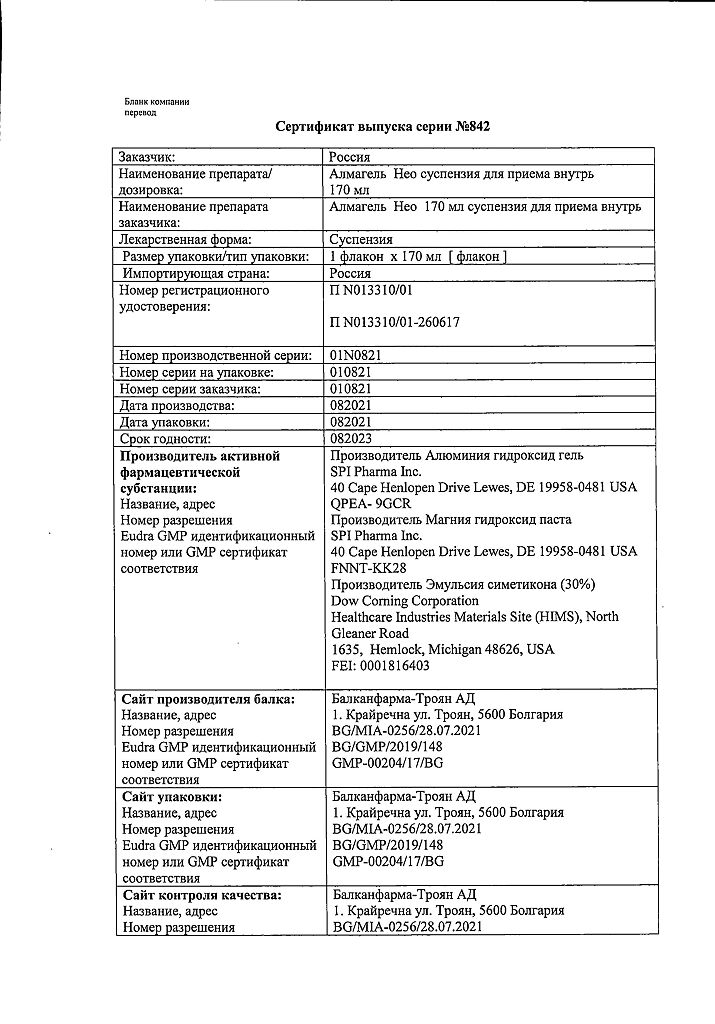
Almagel Neo, 170 ml suspension
€9.48 €8.00
EAN: 3800009121112
SKU: 205418
Categories: Medicine, Stomach, intestines, liver, Ulcer and gastritis
Description
Pharmacotherapeutic group: antacidal agent + diuretic.
ATX code: A02AF02
Pharmacological properties
Pharmacodynamics
A combined remedy whose action is due to its constituent components. It has antacid, adsorptive, sedative and diarrheal action. Algeldrat (aluminum hydroxide) and magnesium hydroxide neutralize free hydrochloric acid in the stomach, decrease the acidity of gastric juice, bind bile acids. The relaxing effect of magnesium hydroxide balances the ability of algeldrate to slow intestinal motility. Simethicone impedes the formation of gas bubbles and promotes their destruction. The gases released are absorbed by the intestinal walls and eliminated through peristalsis.
Pharmacokinetics
absorption. Due to physiological and chemical inertness, simethicone and other active ingredients are not absorbed into organs and tissues and are excreted unchanged after passing through the gastrointestinal tract (GIT). Absorption of aluminum and magnesium ions in the intestine is low.
Distribution.In normal renal function, the concentration of aluminum and magnesium in the blood does not change. In patients with chronic renal insufficiency, the concentration of aluminum and magnesium in the blood may increase to toxic values as a result of impaired excretion.
Elimation.Extracted through the intestine.
Indications
Indications
• Heartburn (after excessive consumption of ethanol, nicotine, coffee, taking medications, improper diet).
• Stomach pain.
• Acute gastritis; chronic gastritis with increased and normal secretory function of the stomach (in the acute phase).
• Peptic ulcer of the stomach and duodenum (in the acute phase).
• Symptomatic ulcers of the gastrointestinal tract of various origins.
• Erosion of the mucous membrane of the upper gastrointestinal tract.
• Gastroesophageal reflux, reflux esophagitis.
• Acute duodenitis, duodenogastric reflux.
• Acute pancreatitis, exacerbation of chronic pancreatitis.
• Flatulence.
• Fermentative or putrefactive dyspepsia.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antacid + carminative.
ATX code: A02AF02
Pharmacological properties
Pharmacodynamics
A combined product whose effect is determined by its constituent components. It has an antacid, adsorbent, enveloping, carminative effect. Algeldrate (aluminum hydroxide) and magnesium hydroxide neutralize free hydrochloric acid in the stomach, reduce the acidity of gastric juice, and bind bile acids. The laxative effect of magnesium hydroxide balances the ability of algeldrate to slow down intestinal motility. Simethicone inhibits the formation of gas bubbles and promotes their destruction. The gases released during this process are absorbed by the intestinal walls and removed from the body through peristalsis.
Pharmacokinetics
Suction. Simethicone and other active ingredients, due to physiological and chemical inertness, are not absorbed into organs and tissues and, after passing through the gastrointestinal tract (GIT), are excreted unchanged. Absorption of aluminum and magnesium ions in the intestines is low.
Distribution. With normal kidney function, the concentration of aluminum and magnesium in the blood does not change. In patients with chronic renal failure, the concentration of aluminum and magnesium in the blood may increase to toxic levels as a result of impaired excretion.
Excretion. Excreted through the intestines.
Special instructions
Special instructions
The interval between taking Almagel® Neo and other medications should be 1-2 hours.
One measuring spoon (5 ml) of the drug contains 0.113 g of ethyl alcohol (the contents of 1 sachet (10 ml) contains 0.226 g of ethyl alcohol), as a result of which complications may occur in patients with liver and brain diseases, in those suffering from alcoholism and epilepsy, in pregnant women and children under 18 years of age.
The daily dose of the drug (8 – 12 measuring spoons or the contents of 4-6 sachets) contains 0.904 g – 1.356 g of ethyl alcohol, respectively.
The maximum daily dose of the suspension (12 scoops or 6 sachets) contains 1.356 g of ethyl alcohol.
One scoop (5 ml) of Almagel® Neo contains 0.475 g of sorbitol, which is contraindicated in case of congenital fructose intolerance and can cause stomach irritation and diarrhea.
During long-term therapy with the drug, it is necessary to maintain a sufficient intake of phosphorus from food, since aluminum hydroxide is able to bind phosphates and delay their absorption in the gastrointestinal tract. In addition, therapy is associated with increased calcium elimination and imbalance of calcium and phosphorus, which can lead to osteomalacia, manifesting as general weakness and bone pain.
Impact on the ability to drive vehicles and machinery
Does not have a negative impact on the ability to drive vehicles and operate mechanisms that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Algeldrat, Magnesium hydroxide, Simethicone
Composition
Composition
5 ml (one scoop) of suspension contains:
active ingredients: aluminum hydroxide gel (in terms of aluminum hydroxide) 340.00 mg, magnesium hydroxide paste (in terms of magnesium hydroxide) 395.00 mg, simethicone (simethicone emulsion) (in terms of dimethicone) 36.00 mg;
excipients: hydrogen peroxide solution 30% – 0.495 mg, sorbitol – 474.60 mg, sodium saccharinate – 1.13 mg, hyethylose – 5.65 mg, citric acid monohydrate – 5.65 mg, ethyl parahydroxybenzoate – 7.90 mg, propyl parahydroxybenzoate – 3.40 mg, propylene glycol – 113.00 mg, macrogol 4000 – 452.00 mg, orange flavor – 2.26 mg, ethanol 96% – 113.00 mg, purified water up to 5 ml.
10 ml (1 sachet) of suspension contains:
active ingredients: aluminum hydroxide gel (in terms of aluminum hydroxide) 680.00 mg, magnesium hydroxide paste (in terms of magnesium hydroxide) 790.00 mg, simethicone (simethicone emulsion) (in terms of dimethicone) 72.00 mg;
excipients: hydrogen peroxide solution 30% – 0.990 mg, sorbitol – 949.20 mg, sodium saccharinate – 2.26 mg, hyethylose – 11.30 mg, citric acid monohydrate – 11.30 mg, ethyl parahydroxybenzoate – 15.80 mg, propyl parahydroxybenzoate – 6.80 mg, propylene glycol – 226.00 mg, macrogol 4000 – 904.00 mg, orange flavor – 4.52 mg, ethanol 96% – 226.00 mg, purified water up to 10 ml.
Pregnancy
Pregnancy
The use of the drug during pregnancy is contraindicated (see Section “Contraindications”).
Due to the lack of sufficient clinical data, women during breastfeeding should use the drug only after consulting a doctor (see section “With caution”).
Contraindications
Contraindications
• Hypersensitivity to the active substance or any excipient included in the preparation.
• Chronic renal failure.
• Pregnancy.
• Hypophosphatemia.
• Congenital fructose intolerance.
• Alzheimer’s disease.
• Children under 14 years of age due to the lack of data on effectiveness and safety.
With caution
Breastfeeding period, liver cirrhosis, alcoholism, traumatic brain injury, epilepsy, encephalopathy, dementia, children (over 14 years old). In patients with acute renal failure, an increase in the concentration of aluminum and/or magnesium in the blood plasma is possible. Therefore, it should be borne in mind that prolonged use of high doses of the drug can lead to the development of dementia and microcytic anemia. During treatment of patients with acute renal failure, it is necessary to monitor the dynamics of clinical symptoms, ulcer size, the appearance of diarrhea, and the concentration of aluminum and magnesium in the blood serum.
In children (over 14 years of age), the use of magnesium hydroxide can lead to the development of hypermagnesemia, especially with the concomitant presence of acute renal failure or dehydration.
Patients with the diseases listed in this section are advised to consult a doctor before starting to take the drug.
Side Effects
Side Effects
Adverse reactions are systematized in accordance with the World Health Organization (WHO) Classification: very common (≥1/10); often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10000, <1/1000); very rare (< 1/10000); frequency unknown (cannot be determined from available data).
Immune system disorders: frequency unknown – allergic reactions, including bronchospasm.
Metabolic and nutritional disorders: very rarely – hypermagnesemia1.
Nervous system disorders: rarely – intoxication with aluminum and/or magnesium (mainly in renal failure); very rarely – dementia, worsening severity of Alzheimer’s disease.
Gastrointestinal disorders: uncommon: changes in taste sensations; very rarely – diarrhea; frequency unknown – constipation, abdominal pain1.
Nausea and vomiting were also observed. With long-term use in high doses – hypophosphatemia, hypocalcemia, hypercalciuria, osteomalacia, osteoporosis, hypermagnesemia, hyperaluminemia, encephalopathy, nephrocalcinosis, renal dysfunction. In patients with concomitant renal failure – thirst, decreased blood pressure, hyporeflexia.
If any of the side effects indicated in the instructions get worse or you notice any other side effects not listed in the instructions, tell your doctor.
1 Observed with long-term use of magnesium hydroxide in patients with renal failure.
Interaction
Interaction
The drug Almagel® Neo reduces and slows down the absorption of digoxin, indomethacin, salicylates, chlorpromazine, phenytoin, H2-histamine receptor blockers, beta-blockers, diflunisal, ketoconazole and itraconazole, isoniazid, tetracycline antibiotics and quinolones, azithromycin, cefpodoxime, pivampicillin, rifampicin, indirect anticoagulants, barbiturates, fexofenadine, dipyridamole, zalcitabine, chenodeoxycholic and ursodeoxycholic acids, penicillamine and lansoprazole.
M-anticholinergic blockers, by slowing down gastric emptying, enhance and prolong the effect of the drug.
An increase in urinary pH secondary to the use of magnesium hydroxide may alter the excretion of certain drugs. For example, an increase in the excretion of salicylates was noted.
If you are taking other medications, you should consult your doctor before taking Almagel® Neo.
Overdose
Overdose
Symptoms: despite the fact that Almagel® Neo is partially absorbed, prolonged use of high doses can lead to the development of hypermagnesemia (increased magnesium levels in the blood), which is characterized by fatigue, facial flushing, exhaustion, muscle weakness and inappropriate behavior.
Signs of metabolic alkalosis may also be observed: changes in mood or mental activity, numbness or muscle pain, nervousness and fatigue, decreased breathing rate, unpleasant taste sensations.
Urgent measures: it is necessary to immediately take measures to quickly remove the drug – gastric lavage, stimulation of vomiting, taking activated charcoal.
In case of overdose, symptomatic therapy is required.
Storage conditions
Storage conditions
At a temperature not higher than 25 oC.
Do not freeze!
Keep out of the reach of children!
Shelf life
Shelf life
2 years.
Do not use after expiration date.
Manufacturer
Manufacturer
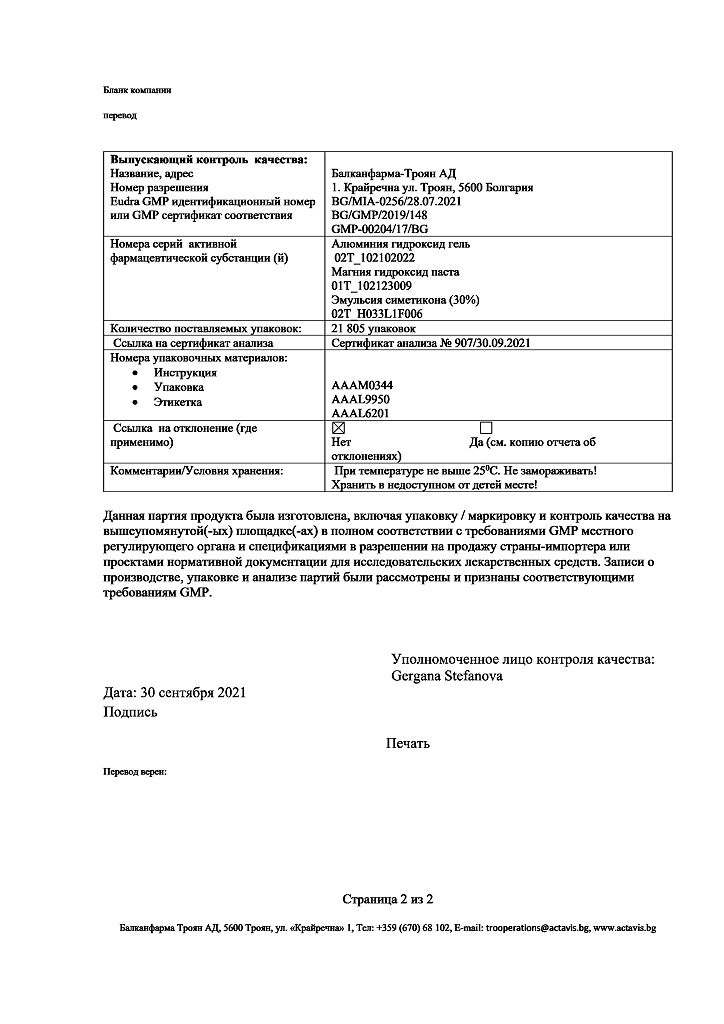
Balkanpharma-Troyan AD, Bulgaria
Additional information
| Shelf life | 2 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At temperatures not exceeding 25 C. Do not freeze! Keep out of reach of children! |
| Manufacturer | Balkanpharma – Troyan AD, Bulgaria |
| Medication form | oral suspension |
| Brand | Balkanpharma – Troyan AD |
Other forms…
Related products
Buy Almagel Neo, 170 ml suspension with delivery to USA, UK, Europe and over 120 other countries.