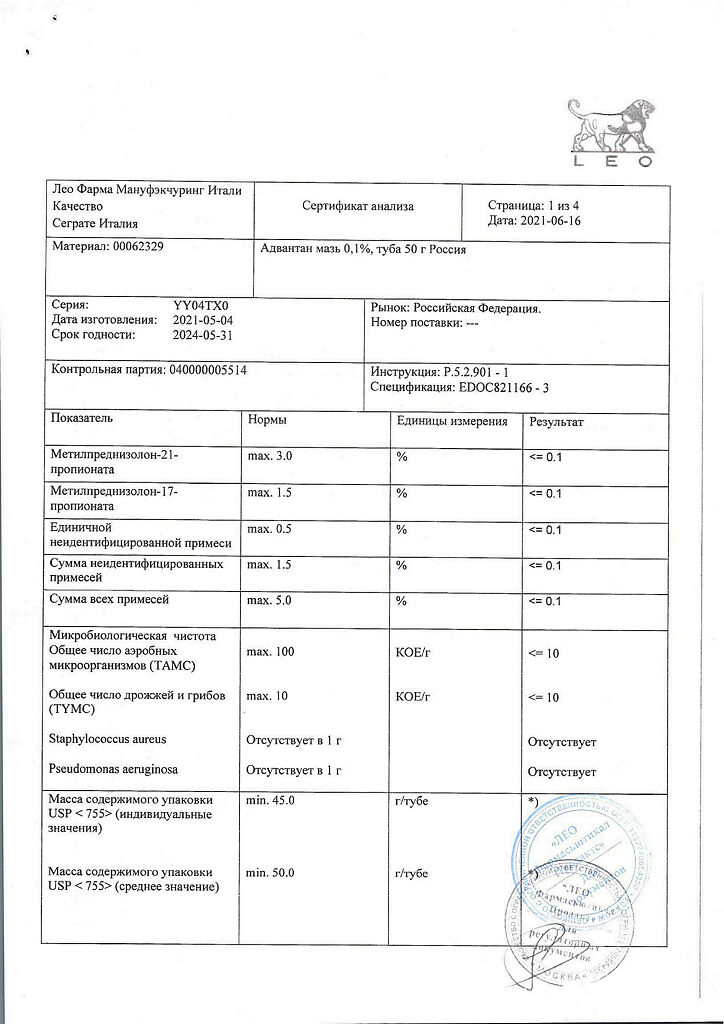
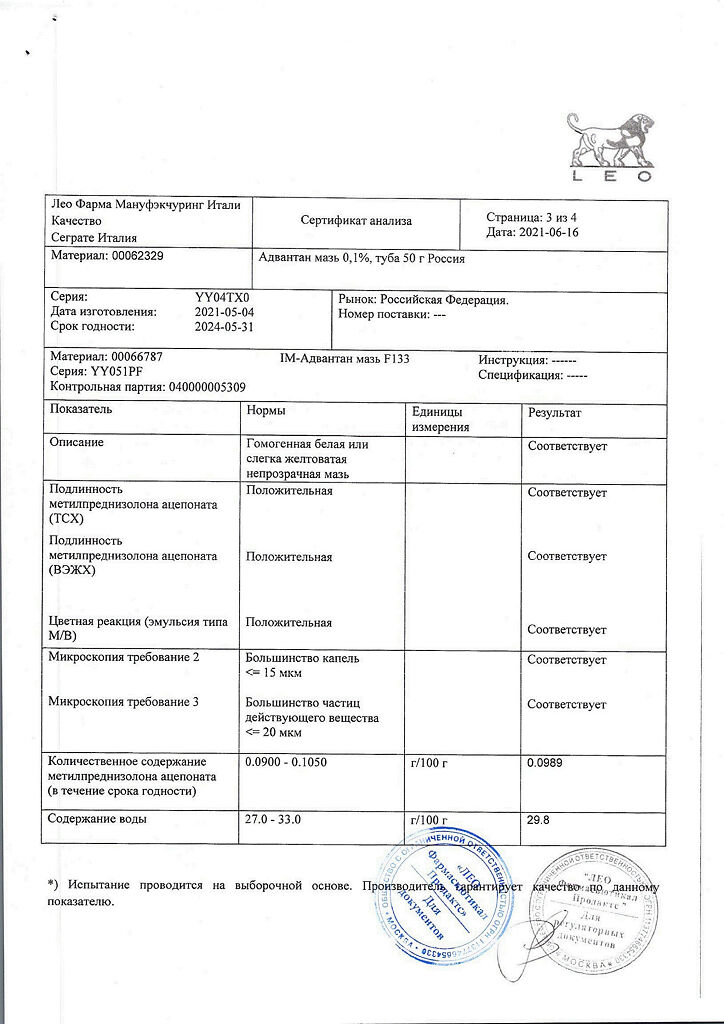
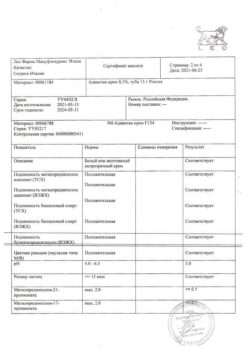
No products in the cart.
Advantan, ointment 0.1% 50 g
€39.68 €33.07
Description
The oral GCS is a nonhalogenated synthetic steroid. When used externally, Advantan suppresses inflammatory and allergic skin reactions as well as reactions associated with increased proliferation, which leads to a reduction of objective symptoms (erythema, edema, lichenification) and subjective sensations (itching, irritation, pain).
When methylprednisolone aceponate is used externally, the systemic effect is expressed minimally in both humans and animals. After multiple application of Advantan on large surfaces (40-60% of the skin surface), as well as when used under the occlusive dressing no adrenal disturbances are observed: the level of cortisol in the blood plasma and its circadian rhythm remain within normal limits, there is no reduction of cortisol level in daily urine.
In clinical studies, no skin atrophy, telangiectasia, striae, or acne-like rashes have been observed in adults and children (including infants) up to 12 weeks when Advantan is used in the form of an ointment, cream, fatty ointment, or emulsion.
Methylprednisolone aceponate (especially its main metabolite, 6α-methylprednisolone-17-propionate) binds to intracellular glucocorticoid receptors. The steroid-receptor complex binds to specific sites of DNA, thus causing a series of biological effects.
In particular, binding of the steroid-receptor complex to DNA leads to induction of macrocortin synthesis. Macrocortin inhibits the release of arachidonic acid and thus the formation of inflammatory mediators such as prostaglandins and leukotrienes.
The inhibition of vasodilatory prostaglandin synthesis by GCS and potentiation of the vasoconstrictor effect of adrenaline leads to a vasoconstrictor effect.
Indications
Indications
Inflammatory skin diseases sensitive to therapy with topical corticosteroids:
— atopic dermatitis, neurodermatitis, childhood eczema;
– true eczema;
– microbial eczema;
– occupational eczema;
– dyshidrotic eczema;
– simple contact dermatitis;
– allergic (contact) dermatitis.
Pharmacological effect
Pharmacological effect
GCS for external use is a non-halogenated synthetic steroid. When applied externally, Advantan suppresses inflammatory and allergic skin reactions, as well as reactions associated with increased proliferation, which leads to a reduction in objective symptoms (erythema, swelling, lichenification) and subjective sensations (itching, irritation, pain).
When methylprednisolone aceponate is used externally, the systemic effect is minimal in both humans and animals. After repeated application of Advantan to large surfaces (40-60% of the skin surface), as well as when applied under an occlusive dressing, no dysfunction of the adrenal glands is observed: the level of cortisol in the blood plasma and its circadian rhythm remain within normal limits, and there is no decrease in the level of cortisol in daily urine.
During clinical studies, the use of Advantan in the form of an ointment, cream, fatty ointment or emulsion for up to 12 weeks in adults and up to 4 weeks in children (including young children) did not reveal the development of skin atrophy, telangiectasia, stretch marks and acne-like rashes.
Methylprednisolone aceponate (especially its main metabolite, 6α-methylprednisolone-17-propionate) binds to intracellular glucocorticoid receptors. The steroid receptor complex binds to specific sections of DNA, thereby causing a series of biological effects.
In particular, the binding of the steroid receptor complex to DNA leads to the induction of macrocortin synthesis. Macrocortin inhibits the release of arachidonic acid and, thereby, the formation of inflammatory mediators such as prostaglandins and leukotrienes.
GCS inhibition of the synthesis of vasodilating prostaglandins and potentiation of the vasoconstrictor effect of adrenaline leads to a vasoconstrictor effect.
Special instructions
Special instructions
In the presence of bacterial dermatoses and/or dermatomycosis, in addition to Advantan therapy, it is necessary to carry out specific antibacterial or antifungal treatment.
Avoid contact of the drug with the eyes.
As with the use of systemic corticosteroids, glaucoma may develop with topical use of corticosteroids (for example, after high doses or very long-term use, use of occlusive dressings, or application to the skin around the eyes).
Does not affect the ability to drive vehicles or operate machinery.
Active ingredient
Active ingredient
Methylprednisolone aceponate
Composition
Composition
1 g ointment contains:
methylprednisolone aceponate 1 mg.
Excipients:
white soft paraffin – 350 mg,
liquid paraffin – 239 mg,
white beeswax – 40 mg,
emulsifier Dehimuls E – 70 mg,
purified water – 300 mg.
Pregnancy
Pregnancy
If it is necessary to use the drug Advantan during pregnancy and lactation, you should carefully weigh the expected benefits of therapy for the mother and the potential risk for the fetus or infant.
In this category of patients, it is not recommended to use the drug for a long time on large surfaces of the skin.
Nursing mothers should not apply the drug to the mammary glands.
Contraindications
Contraindications
– skin tuberculosis and skin manifestations of syphilis in the area of application of the drug;
– viral skin lesions in the area where the drug is applied (for example, with chickenpox, herpes zoster);
— rosacea, perioral dermatitis in the area of application of the drug;
— skin manifestations of a reaction to vaccination;
– children up to 4 months of age;
– hypersensitivity to the components of the drug.
Side Effects
Side Effects
The drug is usually well tolerated.
Very rarely (in less than 0.01% of cases) local reactions may be observed, such as itching, burning, erythema, and the formation of a vesicular rash.
If the drug is used for more than 4 weeks and/or on an area of 10% or more of the body surface, the following reactions may occur: skin atrophy, telangiectasia, stretch marks, acneiform skin changes, systemic effects due to corticosteroid absorption.
In clinical studies, none of the above side effects were noted when using Advantan for up to 12 weeks in adults and up to 4 weeks in children.
In rare cases (0.01-0.1%), folliculitis, hypertrichosis, perioral dermatitis, skin depigmentation, and allergic reactions to one of the components of the drug may occur.
Interaction
Interaction
Drug interactions with Advantan have not been described.
Overdose
Overdose
When studying the acute toxicity of methylprednisolone aceponate, no risk of acute intoxication was identified with excessive single external use (applying the drug over a large area under conditions favorable for absorption) or unintentional ingestion.
Symptoms: with excessively long and/or intensive local use of GCS, skin atrophy (thinning of the skin, telangiectasia, striae) may develop.
Treatment: drug withdrawal.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
LEO Pharma Manufacturing Italy S.r.l., Italy
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | LEO Pharma Manufacturing Italy S.r.l., Italy |
| Medication form | topical ointment |
| Brand | LEO Pharma Manufacturing Italy S.r.l. |
Other forms…
Related products
Buy Advantan, ointment 0.1% 50 g with delivery to USA, UK, Europe and over 120 other countries.