No products in the cart.
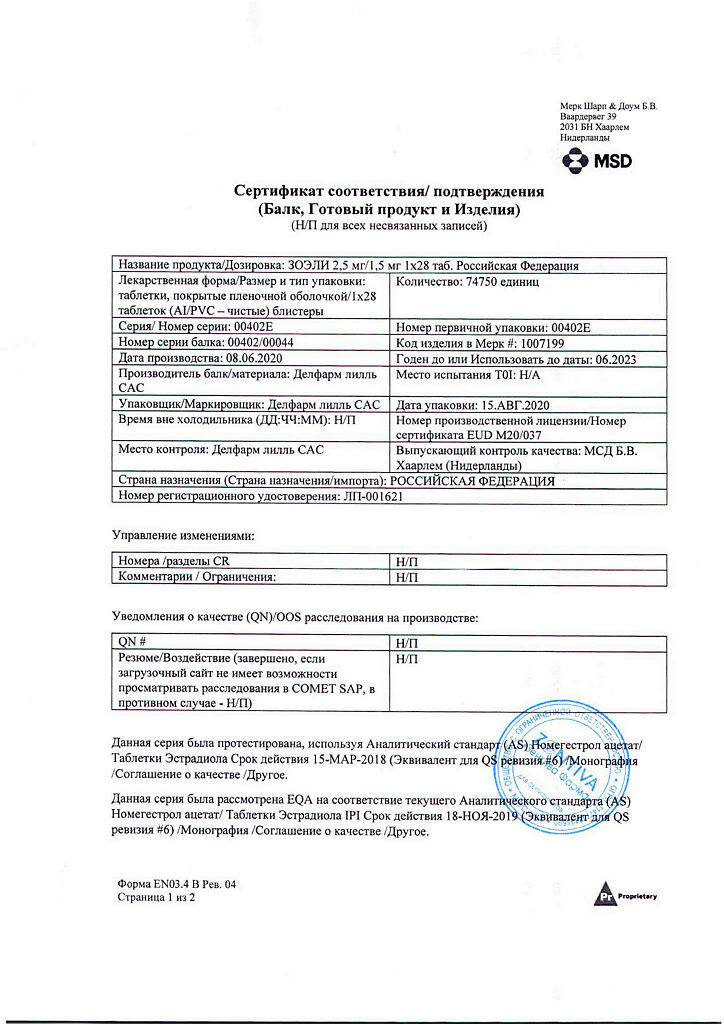
Zoeli, 2.5mg+1.5mg 28 pcs.
€38.46 €32.05
Description
Pharmacodynamics
Nomegestrol acetate is a highly selective progestagen derived from the natural steroid hormone progesterone. Nomegestrol acetate has a pronounced affinity for the human progesterone receptor, has antigonadotropic activity, progesterone receptor-mediated anti-estrogenic activity, moderate anti-androgenic activity and no estrogenic, androgenic, glucocorticoid and mineralocorticoid activity.
Zoeli® contains 17β-estradiol, a natural estrogen identical to endogenous human 17β-estradiol (E2). Unlike ethinylestradiol, which is included in other combined oral contraceptives (OCs), E2 does not have an ethinyl group in the 17α position. When using Zoeli®, the average concentrations of E2 are comparable to those in the initial follicular phase and the late corpus luteum phase of the menstrual cycle (see subsection “Pharmacokinetics”).
The contraceptive effect of Zoeli® is caused by a combination of various factors, the most important of which are suppression of ovulation and change of cervical secretion viscosity.
In two randomized open comparative studies of efficacy and safety, more than 3,200 women aged 18-50 years took Zoelie® for 13 consecutive cycles and more than 1,000 women took the drospirenone (3 mg) / ethinylestradiol (30 mcg) combination in a 21/7 regimen.
In the group taking Zoeli®, weight gain was reported in 8.6% of women (5.7% in the comparison group), irregular “withdrawal” bleeding (predominantly no “withdrawal” bleeding) was reported in 10.5% of women (0.5% in the comparison group), acne was reported in 15.4% of women (7.9% in the comparison group) (see See side effect section).
The evaluation of Zoeli® treatment for acne showed that most women (73.1%) had no change of condition compared to the condition before treatment, 16.8% of women had improvement, and 10.1% of women had appearance or worsening of acne course. In a clinical trial of Zoeli® in the European Union, Asia, and Australia, the following Perl index scores were calculated for the 18-35 age group: method ineffectiveness, 0.40 (upper limit of 95% confidence interval, 1.03); method ineffectiveness and patient error, 0.38 (upper limit of 95% confidence interval, 0.97).
In a clinical trial of Zoeli® conducted in the United States, Canada, and Latin America, the Perl index scores for the 18-35 age group were as follows: method ineffectiveness, 1.22 (95% confidence interval upper limit, 2.18); method ineffectiveness and patient error, 1.16 (95% confidence interval upper limit, 2.08).
In a randomized, open-label study, 32 women received Zoeli® for 6 cycles. After discontinuation of Zoeli®, ovulation was restored an average of 20.8 days after the last pill, with the earliest ovulation date recorded on day 16.
Folic acid is an important vitamin in early pregnancy. While taking Zoeli®, plasma concentrations of folic acid do not change and remain at baseline levels for 6 consecutive months of the drug. In randomized open comparative trial of 2 years in women aged 21-35 years old taking Zoelie® it was observed no clinically significant effect of Zoelie® on bone mineral density.
A randomized, open-label, comparative, multicenter study was conducted to evaluate the effect of Zoeli® on blood clotting parameters, lipid profile, carbohydrate metabolism, adrenal and thyroid function, and androgen concentration.
Sixty women aged 18-50 years took Zoeli® for 6 consecutive cycles. In clinical studies it was found that insulin resistance and glucose tolerance did not change while taking Zoeli® and no clinically significant effects on lipid metabolism and hemostasis were found. Administration of Zoeli® increased concentrations of thyroxine-binding globulin and corticosteroid-binding globulin (CRBG) transporter proteins. Taking Zoeli® slightly increased the concentration of sex hormone-binding globulin (hSBG) and significantly decreased the concentrations of androstenedione, dehydroepiandrosterone, total and free testosterone. In a clinical study in a group of women (n=32) after 13 cycles of taking Zoeli® no pathological changes were observed at histological examination of the endometrium.
Pharmacokinetics
Nomegestrol acetate
Absorption
Nomegestrol acetate is rapidly absorbed after oral administration. After a single dose, the maximum plasma concentration is about 7 ng/ml and is reached after 2 hours. Absolute bioavailability after a single dose is 63%. Food has no clinically significant effect on the bioavailability of nomegestrol acetate.
Distribution
Nomegestrol acetate actively binds to albumin (97-98%) but does not bind to hGH or CRC. The apparent distribution volume of nomegestrol acetate in the equilibrium state is 1645 ± 576 L.
Metabolism
Nomegestrol acetate is metabolized to several inactive hydroxylated metabolites by liver cytochrome P450 isoenzymes, primarily CYP3A4 and CYP3A5; CYP2C8 and CYP2C19 isoenzymes may also participate in metabolism.
Nomegestrol acetate and its hydroxylated derivatives undergo pronounced phase 2 metabolism to form glucuronide and sulfate conjugates. Clearance in the equilibrium state is 26 l/h.
Elimination
The elimination half-life (t1/2) in the equilibrium state is 46 h (28 to 83 h). The elimination half-life of the metabolites has not been established. Nomegestrol acetate is excreted by the kidneys and through the intestine. About 80% of the dose is excreted by the kidneys and through the intestine within 4 days. Nomegestrol acetate is almost completely eliminated within 10 days. Intestinal excretion exceeds that of the kidneys.
The linearity
The linearity of pharmacokinetics depending on the dose was noted in the range of 0.625-5 mg (evaluated in women of reproductive and postmenopausal age).
The equilibrium state
HGH has no effect on the pharmacokinetics of nomegestrol acetate. The equilibrium state is reached after 5 days. The average concentration in the equilibrium state is 4 ng/ml. Maximum plasma concentration of nomegestrol acetate is about 12 ng/ml and is reached 1.5 h after drug administration.
In vitro
In vitro nomegestrol acetate has no significant inducing or inhibitory effect on cytochrome P450 isoenzymes and does not interact with glycoprotein P.
Estradiol (E2)
Intake
Estradiol undergoes marked metabolism during “first passage” through the liver after oral administration. Absolute bioavailability is about 1%. Food intake has no clinically significant effect on the bioavailability of estradiol.
Distribution
The distribution of exogenous and endogenous estradiol is similar. Estrogens are actively distributed throughout the body. Their concentrations are usually higher in the target organs of sex hormones. In the blood, estradiol is bound to hGH (37%) and albumin (61%) and only 1-2% of estradiol circulates unbound.
Metabolism
Exogenous estradiol is actively biotransformed after oral administration. The metabolism of exogenous and endogenous estradiol is similar. Estradiol is rapidly converted to several metabolites in the intestine and liver (mainly to estrone), which are subsequently conjugated and subjected to intrahepatic recirculation. There is a dynamic equilibrium between estradiol, estrone, and estrone sulfate through the activity of various enzymes, including estradiol dehydrogenases, sulfotransferases, and arylsulfatases. Estrone and estradiol are oxidized by cytochrome P450 isoenzymes, mainly CYP1A2, CYP1A2 (outside the liver), CYP3A4, CYP3A5, CYP1B1 and CYP2C9.
Extractions
Estradiol is rapidly excreted from the blood. There is a large pool of circulating estrogen sulfates and glucuronides due to metabolism and intrahepatic recirculation. As a result, the half-life of estradiol, adjusted for baseline, varies widely and is 3.6 ± 1.5 h after intravenous administration. Equilibrium state The maximum plasma concentration of estradiol is about 90 pg/ml and is reached 6 h after administration. Average plasma concentrations are 50 pg/ml. These estradiol concentrations correspond to those in the early and late phases of the menstrual cycle.
Pharmacokinetics in special groups
Pharmacokinetics of nomegestrol acetate (primary target) after a single oral administration of Zoeli® was comparable in healthy adolescent girls after menarche and in adult women. Estradiol concentrations (secondary target) in adolescent girls compared to adult women were comparable during the first 24 hours and lower than in adult women after 24 hours. The clinical significance of this result is unknown.
Renal dysfunction
The effect of renal disease on the pharmacokinetics of Zoeli® has not been studied.
Hepatic impairment
The effect of liver disease on the pharmacokinetics of Zoeli® has not been studied. However, in patients with hepatic impairment, impairment of sex hormone metabolism is possible.
Ethnic groups
The pharmacokinetics of the drug have not been specifically studied in ethnic groups.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
For the tablets containing the active ingredients
Active ingredients:
Nomegestrol acetate 2.50 mg,
Estradiol hemihydrate 1.55 mg (equivalent to 1.50 mg estradiol).
Auxiliary substances:
Microcrystalline cellulose 14.00 mg,
crospovidone 2.40 mg,
talc 0.70 mg,
Magnesium stearate 0.70 mg,
Colloidal silica 0.44 mg,
Lactose monohydrate 57.71 mg;
Pill sheath:
Opadray II white (1.6 mg) contains polyvinyl alcohol 0.64 mg, titanium dioxide 0.40 mg, macrogol-3350 0.32 mg, talc 0.24 mg.
For tablets containing no active ingredients (placebo)
Associates:
Microcrystalline cellulose 14.00 mg,
Crospovidone 2.40 mg,
Talc 0.70 mg,
magnesium stearate 0.70 mg,
silicon dioxide colloid 0.44 mg,
lactose monohydrate 61.76 mg;
Pill sheath:
Opadray II Yellow (2.4 mg) contains polyvinyl alcohol 0.96 mg, titanium dioxide 0.58 mg, macrogol-3350 0.48 mg, talc 0.36 mg, iron oxide yellow dye 0.016 mg, iron oxide black dye 0.00024 mg.
How to take, the dosage
How to take, the dosage
The drug is intended to be taken orally.
How Zoeli® should be taken
The tablets are taken daily at the same time of day, regardless of meals, in the order shown on the package, with a small amount of water if necessary. One tablet daily for 28 consecutive days should be taken. The intake should be started with the white tablets containing the active ingredients.
The white tablets containing the active ingredients are taken for the first 24 days. During the next 4 days, yellow tablets containing no active ingredients (placebos) are taken. Each successive package of tablets should be started the day after the last tablet from the previous package, regardless of the presence or absence of withdrawal bleeding. Bleeding “cancellation” usually begins 2-3 days after taking the last white tablet and may not stop by the beginning of taking the tablets from the next package. For more information, see the “Special Instructions” subsection “Changes in menstrual pattern”.
Particular Patient Groups
There are no data on use in patients with renal impairment, but the effect of renal impairment on excretion of nomegestrol acetate and estradiol is unlikely.
Hepatic impairment
The effect of liver disease on the pharmacokinetics of Zoeli® has not been studied. However, because in patients with hepatic impairment a worsening of sex hormone metabolism is possible, the use of Zoelie® in this group of patients is not recommended until the liver function parameters have normalized (see section “Contraindications”).
How to start Zoeli®
Hormonal contraceptives have not been used in the previous cycle
The pills should be started on the first day of the woman’s menstrual cycle (the first day of menstrual bleeding). In this case the use of additional contraceptives is not required. You may also start taking the pills on days 2-5 of your cycle. In this case during the first 7 days of taking the pills it is recommended to use an additional barrier method of contraception.
To switch from a combined hormonal contraceptive (OC, vaginal ring, or transdermal patch)
A woman should preferably start Zoeli® the day after taking the last tablet containing the active ingredient, but no later than the day after the normal cycle interval or placebo pills are completed. If a woman has used a vaginal ring or a transdermal patch, it is advisable to start Zoelie® on the day it is removed, but no later than the day a new ring or patch should have been put on.
Transitioning from progestogen-only products (pills, implants, injectable forms, or hormone-containing intrauterine systems (IUDs))
A woman can stop taking progestogen-only pills any day and begin taking Zoeli® the next day. The implant or IUD may be removed on any day. In this case, Zoeli® should be started on the day it is removed. If the woman has received injections, Zoelie® should be started on the day of the next injection. In all these cases the woman is recommended to use an additional barrier method of contraception during the first 7 days of taking the tablets containing the active ingredients.
After a first trimester abortion,
a woman can begin taking the drug immediately. In this case, there is no need for an additional method of contraception.
After delivery or second trimester abortion
For breastfeeding women, see “Use during pregnancy and breastfeeding.
A woman should start taking the drug between day 21 and 28 after giving birth or having a second trimester abortion. If starting the drug later, an additional barrier method of contraception is recommended for the first 7 days of taking the pills. However, if after childbirth or abortion sexual intercourse has already occurred, it is necessary to rule out pregnancy or to wait for the first menstrual period before starting Zoeli® therapy.
What to do if you miss the pills
The recommendations below apply only to missing the white pills containing the active ingredients.
If a woman takes her next pill less than 12 hours late, the contraceptive effect is not reduced. The woman should take the pill as soon as she remembers it. Subsequent pills should be taken at the usual time.
If a woman takes the active pill more than 12 hours late, the contraceptive effect may decrease. It is wise to follow two rules if you miss taking the pill:
Recommendations for skipping pills
If one white pill containing the active ingredient is missed
The contraceptive effect is not reduced. A woman should take the last missed white pill as soon as she remembers it, even if she has to take two pills at the same time. The pills should then be taken as usual. No additional contraceptive measures are needed.
If two or more white pills are missed
If after missing two or more white pills containing the active ingredient there was no bleeding “cancellation” while taking the yellow placebo pills, pregnancy should be ruled out (see also Special Instructions, subsection “Changes in menstrual pattern”).
Days 1-7
The woman should take the last missed white pill as soon as she remembers it, even if she has to take two pills at the same time. Then the pills should be taken as usual. In this case, a barrier method of contraception should be used during the first week of taking the white pills continuously. If sexual intercourse took place in the previous 7 days, the possibility of pregnancy should be considered.
Days 8-17
The woman should take the last white pill she missed as soon as she remembers, even if she has to take two pills at the same time. Then the pills should be taken as usual. You must use a barrier method of contraception for the next 7 days of taking the white pills.
Days 18-24
The risk of decreased contraceptive benefit increases as you get closer to taking the yellow placebo pills. However, changing your pill regimen avoids a decrease in contraceptive effect. A woman should take the last white pill she missed as soon as she remembers, even if she has to take two pills at the same time. Do not take more than two white pills containing the active ingredients at the same time. During the next 7 days of taking white pills it is necessary to use a barrier method of contraception, and to start the next package immediately after finishing the white pills from the previous package, i.e. the woman should not take the yellow placebo pills. In this case, bleeding “cancellation” usually occurs while taking the yellow pills from the next package, but “breakthrough” bleeding or “smeared” discharge may occur while taking the white pills.
If a woman is unsure of the number of pills she missed or the color of the pills and therefore does not know what recommendations she should follow, a barrier method of contraception should be used until after the woman has taken the white pills for 7 consecutive days.
If you miss taking the yellow placebo pills
The contraceptive effect is not reduced. A woman can choose not to take the yellow pills from the last (fourth) row of the blister. However, missed pills should be discarded to avoid inadvertently increasing the duration of the placebo phase.
Recommendations in case of gastrointestinal disorders
In case of gastrointestinal disorders (such as vomiting or diarrhea), absorption of the drug may not be complete, so additional contraceptive measures should be used.
If vomiting occurs within 3 to 4 hours of taking the pill, it should be considered missed. If one white pill is missed, the contraceptive effect is not reduced. If vomiting develops again the next day or days, the recommendations for skipping two or more pills should be followed (see “Recommendations for Skipping the Pill”). If a woman does not want to change her regular pill regimen, she should take an additional white pill or pills from a different package.
How to delay or delay menstrual bleeding
To delay menstrual bleeding, a woman should continue taking the white pills from the other package without taking the yellow pills. The white pills in the second pack can continue to be taken until they run out. After the end of taking yellow tablets from the second pack it is necessary to resume taking Zoeli® according to the usual scheme. If the regimen is prolonged, “breakthrough” bleeding or “smeary” discharge may occur.
In order to shift the start day of menstrual bleeding to another day, it is possible to shorten the phase of taking yellow placebo pills (the maximum duration of taking yellow placebo pills is 4 days). The shorter the break, the higher the risk of no menstrual-like bleeding “cancellation” and the occurrence of “breakthrough” bleeding or “smeary” bleeding while taking pills from the second package (as in the case of delayed onset of menstrual-like bleeding).
Interaction
Interaction
To rule out possible interactions, it is necessary to read the instructions for use of concomitant drugs.
The effect of other drugs on Zoeli®
The interaction of oral contraceptives with other enzyme-inducing drugs may lead to “breakthrough” bleeding and/or decrease the effectiveness of contraception.
. Drugs that induce hepatic enzymes (and therefore increase the clearance of sex hormones) include drugs containing phenytoin, phenobarbital, primidone, bosentan, carbamazepine, and rifampicin, medications or herbal preparations containing Hypericum perforatum, and to a lesser extent, medications containing oxcarbazepine, topiramate, felbamate, and griseofulvin. HIV protease inhibitors with inducing activity (e.g. ritonavir and nelfinavir) and non-nucleoside reverse transcriptase inhibitors (e.g. nevirapine and efavirenz) may also affect hepatic metabolism.
A barrier contraception should be used during concomitant administration of drugs that induce microsomal enzymes and for 28 days after their withdrawal. If prolonged treatment with microsomal enzyme inducing drugs is necessary, another method of contraception should be considered.
No drug interaction studies have been conducted for Zoeli®, but two studies were conducted using a combination of nomegestrol acetate and estradiol in higher doses (nomegestrol acetate 3.75 mg + estradiol 1.5 mg) in combination with rifampicin and in combination with ketoconazole in a population of postmenopausal women. Concomitant administration of rifampicin decreases the AUC0-â of nomegestrol acetate by 95% and increases the AUC0-t(last) of estradiol by 25%. Concomitant administration of ketoconazole (single dose of 200 mg) has no effect on estradiol metabolism but increases the maximum concentration (85%) and AUC0-â (115%) of nomegestrol acetate, but these changes are not clinically significant. It has been suggested that similar changes may occur with the use of these drugs in women of reproductive age.
The effect of Zoeli® on other drugs
The oral contraceptives may affect metabolism of other drugs.
Caution should be exercised when using Zoeli® in combination with lamotrigine.
The effect of Zoeli® on laboratory tests
The use of OC may affect the results of some laboratory tests, including biochemical indicators of liver, thyroid, adrenal and renal function, plasma (transport) protein concentrations (e.g., CRP and fractions
lipid/lipidbr> lipids/lipoproteins), carbohydrate metabolism, clotting, and fibrinolysis. These parameters are usually within normal limits.
Special Instructions
Special Instructions
In the presence of any of the following conditions, diseases, risk factors, the benefits of Zoeli® and the possible risks for each individual woman should be evaluated. This should be discussed with the woman prior to starting Zoeli®. If any of these conditions, diseases, or risk factors worsens, worsens, or occurs for the first time, a woman should contact her doctor to decide whether she can continue using Zoeli®.
The following data were obtained in epidemiological studies on the use of OCs containing ethinylestradiol. Zoeli® contains 17β-estradiol; however, the specific guidelines for use of combined contraceptives containing ethinylestradiol are considered to apply to Zoeli® as well.
Vascular events
Contraindications
Contraindications
C OCs should not be used in the presence of any of the following conditions/diseases. There are no epidemiological data on the use of OCs containing 17β-estradiol, but the contraindications for the use of Zoeli® correspond to the contraindications for the use of contraceptives containing ethinylestradiol. In case of any of these conditions/diseases while using Zoeli®, the drug should be stopped immediately.
There are no data about the efficacy and safety of Zoeli® in
adolescent girls less than 18 years of age. The available information on pharmacokinetics can be found in the section “Pharmacokinetics”.
With caution: If any of the following conditions, diseases, or risk factors are present, the benefits of Zoeli® and the possible risks for each individual woman should be evaluated. This should be discussed with the woman before she starts taking Zoeli®. For more information, see the “Special Precautions” section. In cases of worsening, exacerbation or occurrence for the first time of any of these conditions, diseases, risk factors the woman should consult a doctor to decide on the possibility of further use of Zoeli®. Diabetes mellitus without vascular lesions; severe depression or presence of this disease in anamnesis; systemic lupus erythematosus; Crohn’s disease; ulcerative colitis; liver function disorders; hypertriglyceridemia, including a family history; risk factors of coronary heart disease (obesity, arterial hypertension); presence in a family history of venous thrombosis, arterial embolism in brothers, sisters or parents at a young age (see. section “Special indications”).
Side effects
Side effects
The safety of Zoeli® was evaluated in seven multicenter clinical trials lasting up to two years. These studies enrolled 3,490 women aged 18-50 years (total of 3,528 cycles).
The tolerability of Zoeli® is good and the safety profile is similar to that of other OCs. The table lists possible undesirable effects that have been reported with the drug.
The frequency of adverse events is listed in terms:
according to MedDRA (synonyms or related conditions are not listed, but should also be considered).
Metabolic and nutritional disorders: infrequent – increased appetite, fluid retention; rarely – decreased appetite.
Psychiatric disorders: often – decreased libido, depression, mood swings; rarely – increased libido.
Nervous system disorders: often – migraine, headache; rarely – impaired attention.
VIocular disorders: infrequent – contact lens intolerance, dry mucous eyes
vascular disorders: infrequent – “hot flashes”.
Gastrointestinal disorders: frequently – nausea; infrequently – bloating; rarely – dry mouth.
Skin and subcutaneous tissue disorders: very common – acne1; infrequent – hyperhidrosis, alopecia, itching, dry skin, seborrhea; rare – chloasma, hypertrichosis.
Skeletal-muscular system and connective tissue disorders: infrequent – sensation of heaviness.
Disorders of the genital organs and the mammary gland: very often – irregular bleeding “cancellation”; often – heavy acyclic bleeding, heavy menstrual-like bleeding, breast pain, pelvic pain; infrequent – scant menstrual bleeding, mammary gland engorgement, galactorrhea, uterine muscle spasm, premenstrual syndrome, breast masses, dyspareunia, dry vulvar and vaginal mucosa; rarely – foul odor from the vagina, discomfort in the vaginal area.
General disorders and disorders at the injection site: infrequent – irritability, swelling; rarely – feeling of hunger.
Laboratory and instrumental data: often – weight gain; infrequent – increase in liver enzymes activity.
1 – Acne was not a spontaneously reported phenomenon, but a requested one, because it was assessed at each study visit.
In addition to the adverse events noted above, hypersensitivity reactions have been reported with Zoeli® (incidence not established).
The side effects that have occurred while taking OCs containing ethinylestradiol are described in detail in the section “Special Precautions”: venous and arterial thromboembolism, increased blood pressure, hormone-dependent tumors (such as liver tumors, breast cancer), chloasma.
Overdose
Overdose
Repeated administration of Zoeli® at doses that were 5 times higher than recommended and a single administration of nomegestrol acetate at doses that were 40 times higher than recommended were not associated with adverse events.
Symptoms that may occur in overdose: nausea, vomiting, and bloody vaginal discharge.
Treatment: there are no antidotes. Further treatment should be symptomatic.
Pregnancy use
Pregnancy use
Pregnancy
The use of Zoeli® is contraindicated during pregnancy. If pregnancy occurs during the use of Zoeli® , the drug should be discontinued.
The majority of epidemiological studies have found no increased risk of birth defects in children whose mothers took OCs containing ethinylestradiol before pregnancy. No teratogenic effects have been observed with incidental use of OCs containing ethinylestradiol early in pregnancy. There is limited experience with the use of Zoeli® in pregnant women, which indicates no adverse effects of the drug on the fetus or the newborn.
In studies of the nomegestrol acetate/estradiol combination in laboratory animals, reproductive toxicity has been reported.
The drug Zoeli® is designed to prevent unwanted pregnancy. If a woman wants to stop taking Zoeli® to get pregnant, it should be taken into account that ovulation is restored on average 20.8 days after the last tablet of Zoeli® (see section “Pharmacological properties”, subsection “Pharmacodynamics”).
Breastfeeding
Breastfeeding can affect lactation because it changes the amount and composition of breast milk. Consequently, the use of OCs is not recommended until breastfeeding has stopped completely (an alternative method of contraception should be chosen). Small amounts of contraceptive sex hormones and/or their metabolites may be excreted with breast milk, but there are no data on their adverse effects on the health of the newborn.
Additional information
| Weight | 0.043 kg |
|---|---|
| Shelf life | 3 years |
| Conditions of storage | At 2-30 °C |
| Manufacturer | Delpharm Lille S.a.S., France |
| Medication form | pills |
| Brand | Delpharm Lille S.a.S. |
Related products
Gynecology and Obstetrics
Buy Zoeli, 2.5mg+1.5mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.