No products in the cart.
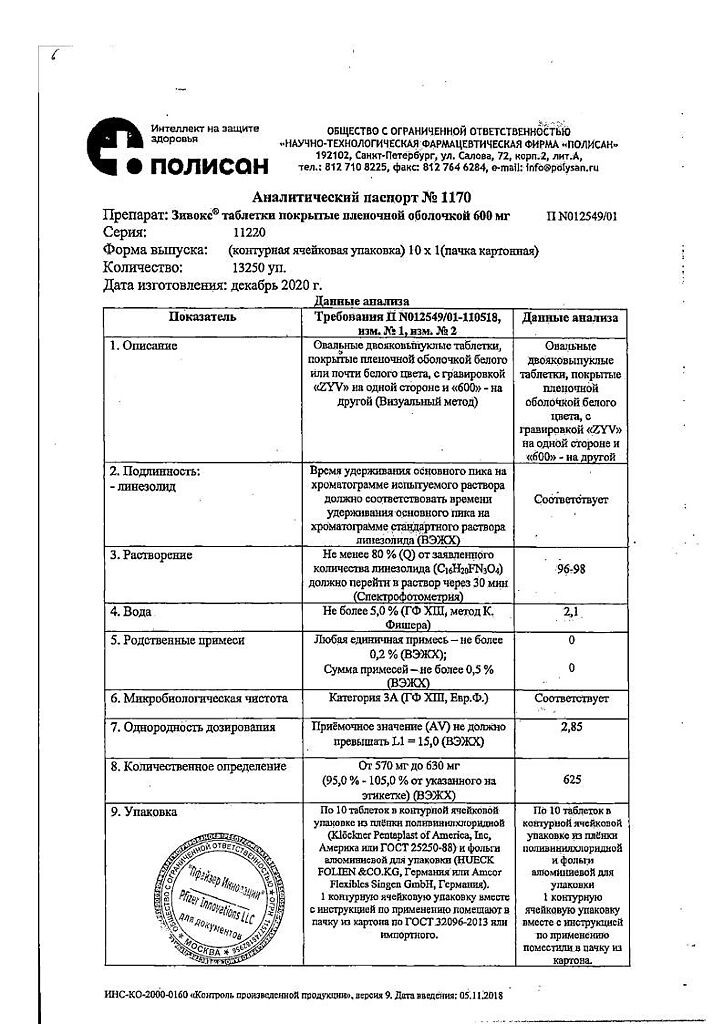
Zivox, 600 mg 10 pcs
€282.52 €244.85
Out of stock
(E-mail when Stock is available)
Description
Pharmacotherapeutic group: Oxazolidinone antibiotic.
ATX code: J01XX08
PHARMACOLOGICAL PROPERTIES
Pharmacodynamics
Linezolid, a synthetic antibacterial drug, belongs to a new class of antimicrobial agents, oxazolidinones, active in vitro against aerobic Gram-positive bacteria, some Gram-negative bacteria and anaerobic microorganisms. Linezolid selectively inhibits protein synthesis in bacteria. Through binding to bacterial ribosomes, it prevents the formation of the functional initiating complex 70S, which is an important component of the translation process during protein synthesis.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
The drug can be taken both with and between meals.
Patients who at the beginning of therapy were prescribed the drug intravenously can be subsequently switched to any dosage form of the drug for oral administration, and no dosage adjustment is required, since the bioavailability of linezolid when taken orally is almost 100%. The duration of treatment depends on the causative agent, the localization and severity of the infection and the clinical effect.
Elderly patients: no dose adjustment is required.
Patients with renal insufficiency: no dose adjustment is required. Because 30% of linezolid is eliminated in hemodialysis within 3 hours, linezolid must be taken after dialysis in patients who need it.
Patients with hepatic impairment: no dose adjustment is required.
Interaction
Interaction
Monoamine oxidase inhibitors
Rifampicin caused decreases in Cmax and AUC of linezolid by an average of 21% and 32%, respectively.
Special Instructions
Special Instructions
In established infections (or suspected infections) caused by concomitant Gram-negative microorganisms, additional use of agents acting on Gram-negative flora is indicated.
Contraindications
Contraindications
WARNING
Patients with renal impairment
Because of the unstudied clinical significance of the two primary metabolites of linezolid in patients with severe renal impairment, linezolid should be used with caution in these patients and only if the anticipated benefit exceeds the potential risk.
Patients with hepatic impairment
There is limited clinical evidence recommending the use of linezolid in such patients only if the anticipated benefit exceeds the potential risk.
Linezolid should be used with caution in patients with systemic infections that pose a risk to life, such as infections associated with venous catheters in intensive care units.
Side effects
Side effects
The frequency of adverse reactions is categorized as follows:
Very frequent: â¥10%
Frequent: â¥1% and < 10%
Infrequent: â¥0.1% and < 1%
Rare: â¥0.01% and < 0.1%
Very rare: < 0.01%
The adverse events associated with taking linezolid are usually mild to moderate in severity. Diarrhea, headache, and nausea are the most common.
Adult patients
Digestive system disorders:
Frequent: diarrhea, nausea, vomiting, constipation, abdominal pain (including spastic), flatulence, oral mucosal candidiasis
Infrequent: change in tongue coloration
Laboratory findings:
Frequent: thrombocytopenia
Infrequent: increased blood triglyceride concentration, increased activity of “liver” enzymes (including alanine aminotransferase (ALT), aspartate aminotransferase (ACT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), lipase, amylase, concentration of total bilirubin and creatinine), increased prolactin concentration
Nervous system disorders:
Frequent: headache, dizziness, seizures
Infrequent: perversion of taste
Central nervous system disorders:
Frequent: insomnia
Urinary system disorders:
Frequent: vaginal candidiasis
Skin disorders:
Frequent: rash
Other:
Frequent: fever
Infrequent: Opportunistic fungal infection
Also noted: increased blood pressure, dyspepsia, itching
Adolescents (12 to 17 years)
Digestive system side:
Frequent: diarrhea, nausea, vomiting, abdominal pain (localized and generalized), liquid stools
Laboratory findings:
Infrequent: eosinophilia, increased blood triglyceride concentration, increased alanine aminotransferase (ALT) activity, lipase, creatinine concentration
Nervous system disorders:
Frequent: headache, vertigo
Skin disorders:
Frequent: rash
Infrequent: itching
Respiratory system disorders:
Frequent: upper respiratory tract infections, pharyngitis, cough
Other:
Frequent: fever, pain of unspecified localization
Spontaneous (postmarketing) findings
Laboratory findings: Reversible myelosuppression (thrombocytopenia, anemia, leukopenia, pancytopenia)
Senses: cases of optic neuropathy, sometimes leading to vision loss (see “Special Indications”).
Allergic reactions: anaphylaxis
Skin disorders: rash, angioedema; bullous skin lesions similar to Stevens-Johnson syndrome
Metabolic disorders: lactoacidosis
Nervous system disorders: peripheral neuropathy, seizures (see “
Digestive system: discoloration of tooth enamel (see “Special Precautions”)
Others: chills, fatigue, serotonin syndrome (see “Interactions with other medicinal products” and “Special Precautions”)
Overdose
Overdose
Pregnancy use
Pregnancy use
No safety studies of linezolid use in pregnancy have been conducted, therefore the use of ZIVOX® in pregnancy is possible only if the estimated benefit to the mother outweighs the potential risk to the fetus.
Additional information
| Weight | 0.028 kg |
|---|---|
| Shelf life | 3 years |
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Pfizer Pharmaceuticals LLC, Puerto Rico |
| Medication form | pills |
| Brand | Pfizer Pharmaceuticals LLC |
Related products
Buy Zivox, 600 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.