No products in the cart.
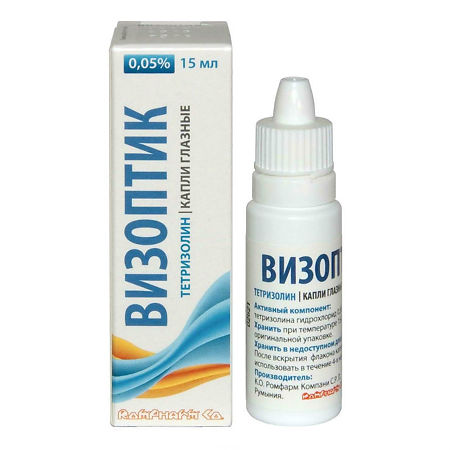
VizOptik, eye drops 0.05% 15 ml
€10.68 €9.34
Description
Alpha-adrenomimetic. It has a vasoconstrictor and anti-edema effect.
When used topically it reduces conjunctival edema, burning sensation, painful mucous membrane of the eyes, lacrimation.
The drying effect develops within a few minutes and lasts for 4-8 hours.
Pharmacokinetics
Systemic absorption is low when used topically.
Indications
Indications
moderate eye irritation: itching, swelling and hyperemia of the conjunctiva, burning, lacrimation, scleral injection caused by chemical and physical factors (dust, cosmetics, swimming in chlorinated water, exposure to bright light or radiation from a computer monitor);
allergic conjunctivitis.
Pharmacological effect
Pharmacological effect
Alpha adrenergic agonist. Has a vasoconstrictor and anti-edematous effect.
When applied topically, it reduces swelling of the conjunctiva, burning sensations, soreness of the mucous membrane of the eyes, and lacrimation.
The vasoconstrictor effect develops within a few minutes and lasts 4-8 hours.
Pharmacokinetics
When applied topically, systemic absorption is low.
Special instructions
Special instructions
VizOptic is advisable to use only for mild eye irritation. If irritation and hyperemia of the conjunctiva are associated with an infectious disease, the presence of a foreign body or chemical injury to the cornea, consultation with an ophthalmologist is necessary.
If within 48 hours after starting the use of eye drops, the symptoms of the disease persist, become more pronounced, or side effects appear, you should stop using the drug and consult an ophthalmologist.
If you experience intense pain in the eyes, headache, blurred vision, sudden appearance of “floating spots” in front of the eyes, redness of the eyes, pain when exposed to light, or double vision, you should immediately consult a doctor.
When wearing contact lenses, they are removed before instillation of the drug and reinstalled 15 minutes after it. Direct contact of eye drops with soft contact lenses should be avoided due to possible disruption of their transparency.
The drug should not be used simultaneously with MAO inhibitors (including phenelzine, nialamide, iproniazid) and within 10 days after stopping their use.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration, speed of psychomotor reactions and good vision (after using eye drops, dilation of the pupil and a feeling of blurred vision are possible).
Active ingredient
Active ingredient
Tetrizoline
Composition
Composition
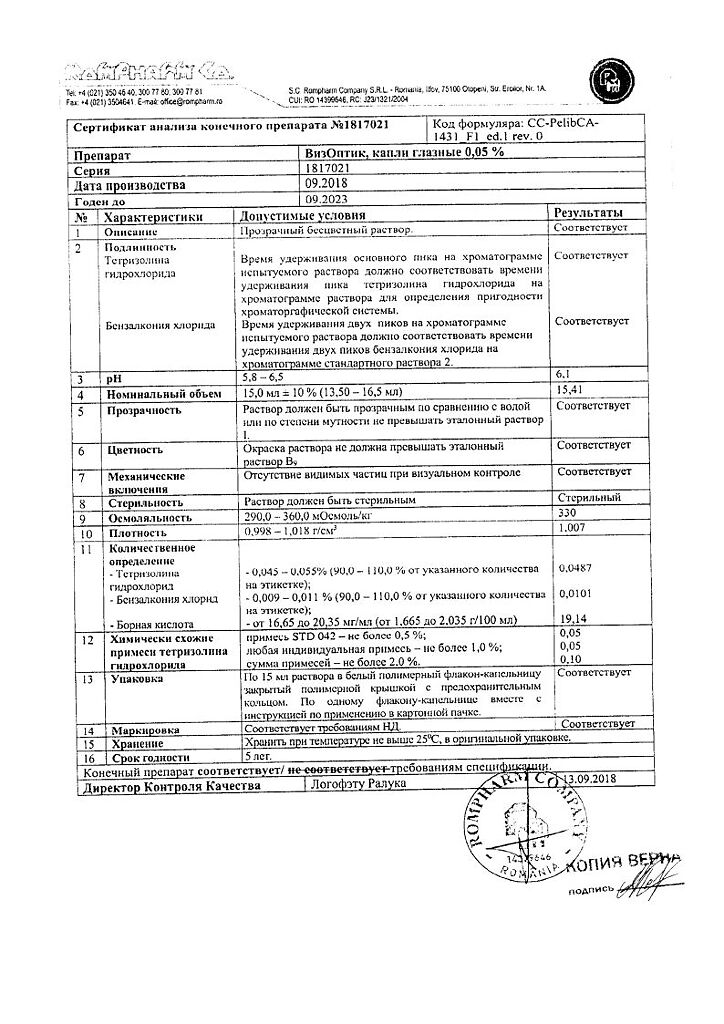
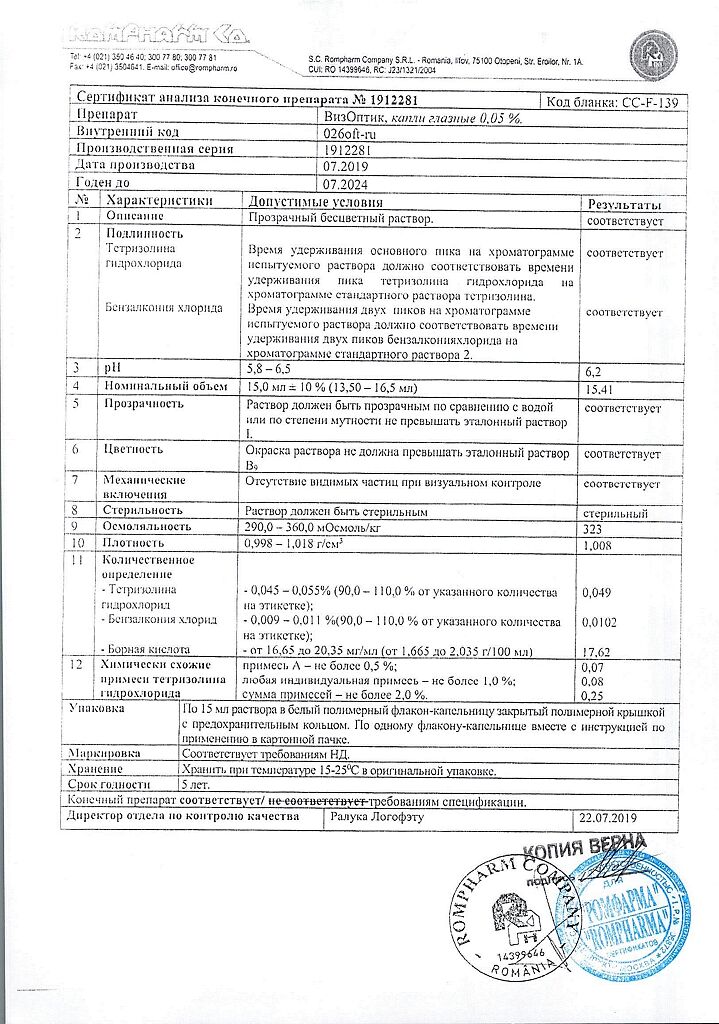
1 ml of eye drops contains:
Active ingredient
Tetrizoline hydrochloride 500 mcg.
Excipients
Boric acid,
sodium tetraborate decahydrate,
disodium edetate dihydrate,
benzalkonium chloride,
purified water.
Pregnancy
Pregnancy
VizOptic is contraindicated for children under 3 years of age. Prescribe with caution during pregnancy and breastfeeding.
Contraindications
Contraindications
Glaucoma.
Endothelial-epithelial dystrophy of the cornea.
Acute cardiovascular failure.
Bacterial eye infection.
Foreign body of the eye.
Individual hypersensitivity to the components of the drug.
Children under 3 years of age.
With caution:
Arterial hypertension.
Arrhythmia.
Aneurysm.
Hyperthyroidism.
Pheochromocytoma.
Diabetes mellitus.
Severe organic diseases of the heart and blood vessels, including:
Coronary heart disease.
During treatment with MAO inhibitors and other drugs that increase blood pressure.
Side Effects
Side Effects
Local
Increased intraocular pressure, burning sensation and reactive hyperemia of the eye.
Rarely – pupil dilation.
Systemic reactions
increased blood pressure, hyperglycemia, cardiac dysfunction, headache, nausea, drowsiness, weakness, tremor, dizziness, insomnia, tachycardia, allergic reactions.
Interaction
Interaction
Concomitant use with MAO inhibitors, tricyclic antidepressants, guanethidine can cause arterial hypertension and tachycardia.
Concomitant use with reserpine, insulin, propranolol, general anesthesia, and atropine increases the risk of cardiovascular effects of tetrizoline.
Concomitant use with guanethidine may cause mydriasis with temporary visual impairment.
Overdose
Overdose
The development of systemic symptoms of overdose is possible when the drug is accidentally or intentionally taken orally or when the dosage is exceeded when applied topically.
Symptoms: pupil dilation, nausea, cyanosis, fever, convulsions, arrhythmias, arterial hypertension, pulmonary edema, dyspnea. Excessive systemic absorption of imidazole derivatives can lead to CNS depression, accompanied by drowsiness, hypothermia, bradycardia, collapse, apnea and coma.
The risk of overdose symptoms associated with drug absorption is high in young children, especially if swallowed. Overdose in children can manifest as depression of the central nervous system, accompanied by drowsiness, excessive sweating, severe hypotension, even shock.
Treatment: activated charcoal, gastric lavage, oxygen inhalation, and the use of antipyretics and anticonvulsants are prescribed. To reduce blood pressure, use phentolamine 5 mg in a 0.9% sodium chloride solution slowly intravenously or 100 mg orally. A specific antidote is unknown.
Storage conditions
Storage conditions
At 15–25 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
K.O.Rompharm Company S.R.L., Romania
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At 15-25 °C |
| Manufacturer | C.O.Rompharm Company S.R.L., Romania |
| Medication form | eye drops |
| Brand | C.O.Rompharm Company S.R.L. |
Related products
Buy VizOptik, eye drops 0.05% 15 ml with delivery to USA, UK, Europe and over 120 other countries.