No products in the cart.
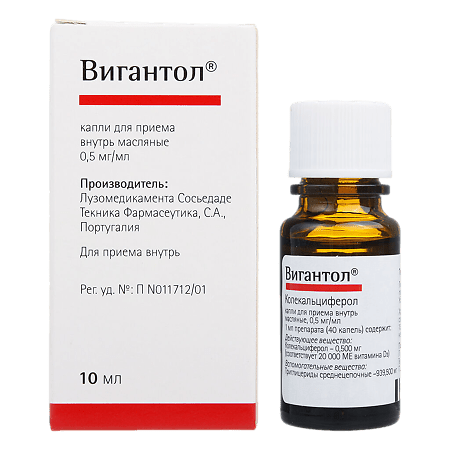
Vigantol, drops 0.5 mg/ml 10 ml
€5.00 €4.71
Description
The purpose of this drug is to regulate calcium and phosphorus metabolism. At the same time vitamin D3 deficiency is compensated, absorption of calcium in the gastrointestinal tract and reabsorption of phosphate in the kidneys is increased. Vigantol also contributes to mineralization of bones, which is necessary for complete functioning of parathyroid glands.
The drug is absorbed from the gastrointestinal tract. Inside the body, the active substance binds with α2-globulins, some part with albumin.
Calciferol accumulation occurs in liver, bone and fat tissue, skeletal muscles, adrenal glands and kidneys, myocardium. Maximum concentration of the substance in the tissue is reached after 4-5 hours, then it decreases slightly, but still remains at the required level for a long time.
As a result of biotransformation in the liver and kidneys are formed inactive metabolites -calciferol and dihydroxycalciferol, the active metabolite calcitriol.
< br>.
Indications
Indications
The main indications for the use of Vigantol are:
rickets;
spasmophilia;
osteomalacia.
Pharmacological effect
Pharmacological effect
The purpose of this drug is to regulate the metabolism of calcium and phosphorus. At the same time, vitamin D3 deficiency is compensated, calcium absorption in the gastrointestinal tract and phosphate reabsorption in the kidneys are enhanced. Vigantol also promotes bone mineralization, which is necessary for the parathyroid glands to function properly.
Absorption of the drug occurs from the gastrointestinal tract. Inside the body, the active substance binds to α2-globulins, some with albumins.
The accumulation of colecalciferol occurs in the liver, bone and adipose tissue, skeletal muscles, adrenal glands and kidneys, and myocardium. The maximum concentration of the substance in tissues is reached after 4-5 hours, then it decreases slightly, but still remains at the required level for a long time.
It has been established that colecalciferol penetrates the placental barrier and is also excreted in breast milk.
As a result of biotransformation in the liver and kidneys, inactive metabolites are formed – calcifediol and dihydroxycolecalciferol, the active metabolite calcitriol.
Special instructions
Special instructions
With pseudohypoparathyroidism, it is necessary to monitor for signs of intoxication. Because with pseudohypoparathyroidism, there may be phases of normal sensitivity to vitamin D; it is necessary to adjust the dose of the drug.
In case of pseudohypoparathyroidism that occurs after surgical treatment of the thyroid gland, it is necessary to stop using the drug as the parathyroid glands are restored to prevent vitamin D intoxication.
Impact on the ability to drive vehicles and operate machinery
Studies on the effect on the ability to drive a vehicle and operate machinery have not been conducted.
Active ingredient
Active ingredient
Colecalciferol
Composition
Composition
Active ingredient:
colecalciferol 500 mcg (20,000 IU)
Excipients:
medium chain triglycerides – 939.5 mg.
Contraindications
Contraindications
hypercalcemia;
hypercalciuria;
calcium nephrourolithiasis;
thyrotoxicosis (possibility of hypersensitivity);
renal osteodystrophy with hyperphosphatemia;
hypervitaminosis D;
hypersensitivity to the components of the drug.
The drug should be prescribed with caution in case of atherosclerosis, heart failure, renal failure, sarcoidosis or other granulomatosis, hyperphosphatemia, phosphate nephrourolithiasis (including a history), organic heart damage, acute and chronic diseases of the liver and kidneys, gastrointestinal diseases (including gastric and duodenal ulcers), hypothyroidism, during pregnancy and breastfeeding, when taking additional amounts of vitamin D3 (for example, as part of other medications).
Side Effects
Side Effects
From the digestive system: constipation, flatulence, nausea, abdominal pain, diarrhea, loss of appetite.
Metabolism: hypercalcemia and hypercalciuria when taking the drug for a long time in high doses, polyuria.
From the musculoskeletal system: myalgia, arthralgia.
From the cardiovascular system: increased blood pressure, arrhythmias.
Allergic reactions: itching, rash, urticaria.
Other: headache, impaired renal function, exacerbation of tuberculosis in the lungs.
Interaction
Interaction
Phenytoin, primidone and barbiturate drugs increase the need for vitamin D3 due to an increase in the rate of biotransformation.
Long-term therapy with the simultaneous use of antacids containing aluminum and magnesium ions increases their concentration in the blood and the risk of intoxication (especially in the presence of chronic renal failure).
Calcitonin, bisphosphonates (including etidronic and pamidronic acid), plicamycin reduce the effect.
Cholestyramine and colestipol reduce the absorption of fat-soluble vitamins from the gastrointestinal tract and require an increase in their dose.
Increases the absorption of phosphorus-containing drugs and the risk of hyperphosphatemia.
When used simultaneously with sodium fluoride, the interval between doses should be at least 2 hours; with oral forms of tetracyclines – at least 3 hours.
With concomitant therapy with GCS, the effectiveness of the drug may be reduced.
Concomitant therapy with cardiac glycosides may increase their toxic potential due to the development of hypercalcemia. In such patients, it is necessary to monitor calcium levels, ECG, and adjust the dose of cardiac glycosides.
Concomitant therapy with benzodiazepine derivatives increases the risk of developing hypercalcemia.
Vitamin D3 can be combined with vitamin D metabolites or analogues only in exceptional cases and under the control of serum calcium levels.
Thiazide diuretics may reduce urinary calcium excretion and, accordingly, increase the risk of developing hypercalcemia. In such patients, it is necessary to constantly monitor the concentration of calcium in the blood.
Rifampicin and isoniazid may reduce the effect of the drug due to an increase in the rate of biotransformation.
Vigantol® does not interact with food.
Overdose
Overdose
Symptoms of hypervitaminosis D3: early (due to hypercalcemia) – constipation or diarrhea, dry oral mucosa, headache, thirst, pollakiuria, nocturia, polyuria, anorexia, metallic taste in the mouth, nausea, vomiting, unusual fatigue, general weakness, adynamia, dehydration, hypercalcemia, hypercalciuria, increased plasma concentrations 25-dihydrocolecalciferol; late – bone pain, cloudiness of urine (appearance of hyaline casts in the urine, proteinuria, leukocyturia), increased blood pressure, skin itching, photosensitivity of the eyes, conjunctival hyperemia, arrhythmia, drowsiness, myalgia, nausea, vomiting, pancreatitis, gastralgia, weight loss; rarely – changes in the psyche (up to the development of psychosis) and mood.
Symptoms of chronic overdose of vitamin D3 (when taken for several weeks or months for adults in doses of 20-60 thousand IU / day, for children – 2-4 thousand IU / day): calcification of soft tissues, kidneys, lungs, blood vessels, increased blood pressure, renal and heart failure (these effects most often occur when hypercalcemia is combined with hyperphosphatemia), growth impairment in children (long-term use at a dose of 1.8 IU/day).
Treatment: in case of acute or chronic overdose, it is necessary to take measures to treat developing hypercalcemia. Depending on the severity of hypercalcemia, the following measures are used: discontinuation of the drug, a low-calcium diet, consumption of large amounts of fluid, administration of corticosteroids, vitamin E, ascorbic acid, retinol, thiamine, pantothenic acid, riboflavin; in severe cases – intravenous administration of 0.9% sodium chloride solution, furosemide, electrolytes, hemodialysis, administration of calcitonin. There is no specific antidote. To avoid overdose, in some cases it is recommended to determine the concentration of calcium in the blood.
Manufacturer
Manufacturer
Luzomedicamenta Sociedad Tecnica Pharmaceutica, Portugal
Additional information
| Manufacturer | Luzomedicamente Sociedade Tecnica Pharmaseutica, Portugal |
|---|---|
| Medication form | oral drops |
| Brand | Luzomedicamente Sociedade Tecnica Pharmaseutica |
Related products
Buy Vigantol, drops 0.5 mg/ml 10 ml with delivery to USA, UK, Europe and over 120 other countries.