No products in the cart.
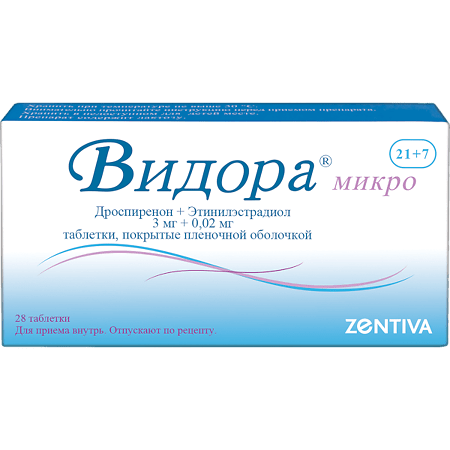
Vidora Micro, 3 mg+0.02 mg 7 pcs.
€21.22 €17.68
EAN: 8437009433836
SKU: 261464
Categories: Contraceptive, Gynecology and Obstetrics, Hormonal, Medicine
Description
Vidora micro is a microdose combined monophasic hormonal contraceptive drug containing ethinyl estradiol and drospirenone. The contraceptive effect of the drug is based on the interaction of various factors, the most important of which are inhibition of ovulation, increasing the viscosity of the cervical secretion, making it impermeable to sperm.
When used correctly, the Perl Index (a measure of the number of pregnancies per 100 women using the contraceptive over the course of a year) is less than 1. If the pill is missed or used incorrectly, the Perl Index may increase.
Drospirenone also has antiandrogenic and weak anti-mineralocorticoid properties at the therapeutic dose. Drospirenone has no estrogenic, glucocorticosteroid or antiglucocorticosteroid activity and has a pharmacological profile similar to that of natural progesterone. With its anti-androgenic activity it helps to reduce sebum production and to improve the clinical course in women with acne (acne vulgaris) which should be considered when choosing a contraceptive method, especially in women with hormone-dependent fluid retention as well as in women with acne and seborrhea.
In combination with ethinylestradiol, it improves the lipid profile and increases the concentration of high-density lipoproteins.
Limiting menstrual bleeding, the drug helps to reduce the severity of pain and volume of menstrual bleeding, thereby reducing one of the risk factors for iron deficiency anemia. In addition, there is evidence that the use of combined oral contraceptives (OCs) reduces the risk of endometrial cancer and ovarian cancer.
Indications
Indications
Oral contraception.
Active ingredient
Active ingredient
Composition
Composition
1 tablet contains drospirenone 3.0mg and ethinylestradiol 0.02mg
Excipients:
Lactose monohydrate – 44 mg,
Corn starch pregelatinized – 38.4 mg,
povidone K30 – 6 mg,
croscarmellose sodium – 1.2 mg,
Polysorbate 80 – 0.9 mg,
Magnesium stearate – 0.5 mg.
Coating composition:
Opadray® II pink – 2.82 mg (partially hydrolyzed polyvinyl alcohol – 1.13 mg, titanium dioxide – 0.68 mg, macrogol-3350 – 0.57 mg, talc – 0.42 mg, iron oxide yellow dye – 0.01 mg, iron oxide red dye – 0.01 mg, iron oxide black dye – 0.001 mg).
Placebo tablets are white.
Associates:
Core:
Lactose anhydrous – 89.5 mg, povidone K30 – 10 mg, magnesium stearate – 0.5 mg.
Shell composition:
Opadray II white – 4 mg (partially hydrolyzed polyvinyl alcohol – 1.6 mg, titanium dioxide – 1 mg, macrogol-3350 – 0.808 mg, talc – 0.592 mg).
How to take, the dosage
How to take, the dosage
The tablets should be taken every day at the same time, with a little liquid if necessary, according to the order listed on the blister pack.
The tablets are taken continuously. One tablet is taken daily for 28 consecutive days.Taking pills from each successive package begins the day after the last tablet from the previous package. Withdrawal bleeding usually begins 2 to 3 days after starting the placebo pills (last line) and may not end until the pills from the next package start.
Overdose
Overdose
Till this time, any experience with drospirenone/ethinylestradiol overdose is not available.
Based on general experience with combined oral contraceptives, the symptoms that can be observed with an overdose of the active pills are nausea, vomiting, and, in young girls, minor bleeding from the vagina.
There are no antidotes and further treatment must be symptomatic.
Similarities
Similarities
Additional information
| Weight | 0.050 kg |
|---|---|
| Manufacturer | Laboratorios Leon Pharma S.A., Spain |
| Medication form | pills |
| Brand | Laboratorios Leon Pharma S.A. |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Buy Vidora Micro, 3 mg+0.02 mg 7 pcs. with delivery to USA, UK, Europe and over 120 other countries.