No products in the cart.
Description
Pharmacological action – vasoconstrictor, anti-allergic, anticongestive.
Pharmacodynamics
Phenylephrine and dimethindene combined drug.
Phenylephrine is a sympathomimetic agent and when applied topically it has moderate vasoconstrictor action (due to stimulation of alpha 1-adrenoreceptors located in the venous vessels of the nasal mucosa), it eliminates edema of the nasal mucosa and its accessory sinuses.
Dimetinden is an anti-allergic agent – an antagonist of histamine H1-receptors; it does not reduce activity of the nasal mucosal epithelium.
Pharmacokinetics
Vibrocil® is for topical use; its activity is independent of the concentration of active substances in the blood plasma.
Indications
Indications
Acute rhinitis (including runny nose due to colds);
allergic rhinitis (including hay fever);
vasomotor rhinitis;
chronic rhinitis;
acute and chronic sinusitis;
acute otitis media (as an auxiliary treatment method).
Preparation for surgical interventions in the nasal area and elimination of swelling of the nasal mucosa and paranasal sinuses after surgical interventions in this area
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: combined antiallergic agent (H1-histamine receptor blocker + alpha-adrenergic agonist).
ATX code: R01AB01.
Pharmacological properties
Pharmacodynamics
Vibrocil is a combination drug containing phenylephrine and dimethindene. Phenylephrine is a sympathomimetic agent; when applied topically, it has a moderate vasoconstrictor effect (due to stimulation of alpha1-adrenergic receptors located in the venous vessels of the nasal mucosa), eliminates swelling of the nasal mucosa and its paranasal sinuses.
Dimetindene is an antiallergic drug – an antagonist of histamine H1 receptors; does not reduce the activity of the ciliated epithelium of the nasal mucosa.
Pharmacokinetics
Vibrocil is intended for topical use, and its activity does not depend on the concentration of active substances in the blood plasma.
Special instructions
Special instructions
Vibrocil should not be used continuously for more than 7 days without consulting a doctor. Long-term or excessive use of the drug can cause tachyphylaxis and the “ricochet” effect associated with the re-development of nasal congestion (rhinitis medicamentosa), leading to the development of a systemic vasoconstrictor effect.
Do not exceed the recommended doses of Vibrocil! Otherwise, manifestations of the systemic vasoconstrictor effect of the drug may develop, especially in children and elderly patients.
Impact on the ability to drive vehicles and operate machinery
No effect.
Active ingredient
Active ingredient
Dimetindene, Phenylephrine
Composition
Composition
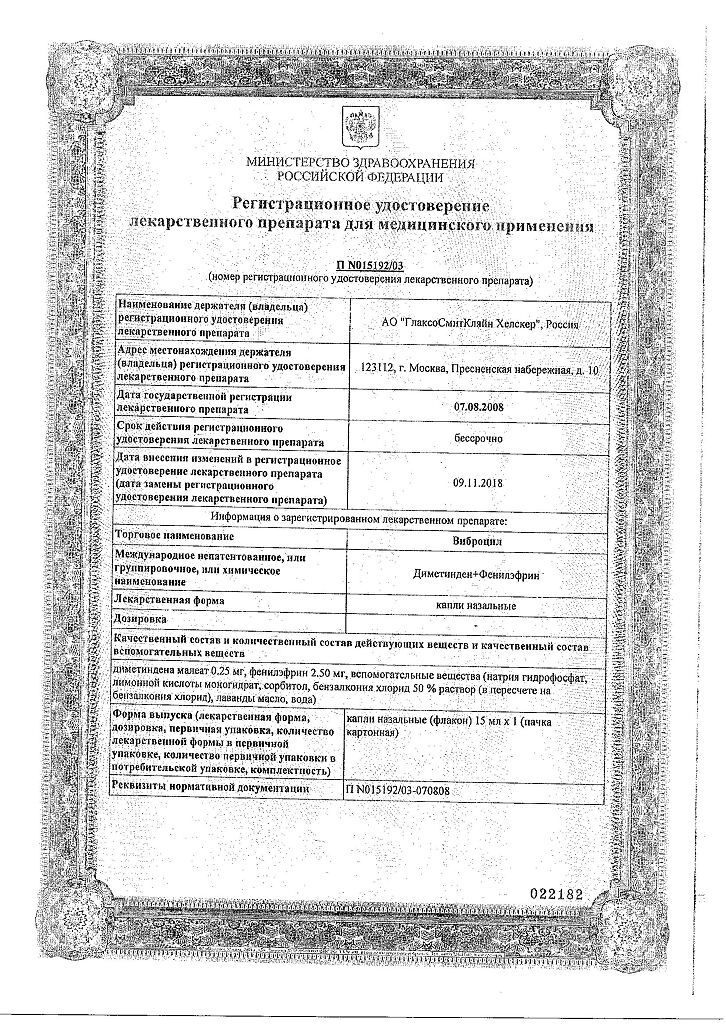
Active ingredients: dimethindene maleate 0.25 mg; phenylephrine 2.5 mg.
Excipients: sodium hydrogen phosphate 4.4 mg, citric acid monohydrate 2.6 mg, sorbitol 35 mg, benzalkonium chloride 50% solution (in terms of benzalkonium chloride) 0.2 mg (0.1 mg), lavender hybrid flower oil 0.2 mg, purified water to 1 ml.
Pregnancy
Pregnancy
Fertility
There are no relevant data on the effects of phenylephrine and dimethindene maleate on fertility in humans. Relevant experimental data in animals regarding the effects of phenylephrine on animal fertility are lacking. Based on animal studies, no adverse effects of dimethindene on animal fertility have been found.
Pregnancy
There are no studies on the use of the drug during pregnancy. Given the possible systemic vasoconstrictor effect of phenylephrine, Vibrocil is not recommended for use during pregnancy.
Phenylephrine
Data on the use of phenylephrine during pregnancy are limited.
Dimetindene maleate
There are no relevant data on the use of dimethindene maleate during pregnancy.
Breastfeeding period
There are no studies on the use of the drug during breastfeeding.
Considering the possible systemic vasoconstrictor effect of phenylephrine, Vibrocil is not recommended for use during breastfeeding.
Phenylephrine
Phenylephrine may be excreted in breast milk.
Dimetindene maleate
Dimetindene maleate may be excreted in breast milk.
Contraindications
Contraindications
Hypersensitivity to phenylephrine, dimethindene maleate or other components of the drug. Atrophic rhinitis (including with foul-smelling discharge – ozena). Taking MAO inhibitors (simultaneously or in the previous 14 days). Angle-closure glaucoma.
Children under 1 year of age.
With caution
Cardiovascular diseases (arterial hypertension, arrhythmias, generalized atherosclerosis), hyperthyroidism, prostate adenoma, diabetes mellitus, bladder neck obstruction (for example, due to prostatic hypertrophy), epilepsy.
As with any local vasoconstrictor, caution should be exercised when prescribing Vibrocil to patients with severe reactions to sympathomimetics, manifested as insomnia, dizziness, tremor, cardiac arrhythmia or increased blood pressure.
Side Effects
Side Effects
According to the World Health Organization (WHO), the frequency of side effects is distributed as follows: very common (≥ 1/10); often (from ≥ 1/100 to < 1/10); uncommon (from ≥ 1/1000 to < 1/100); rare (from ≥ 1/10000 to < 1/1000); very rare (from < 1/10000); frequency is unknown (it is not possible to determine the frequency of occurrence based on available data).
Disorders of the respiratory system, chest and mediastinal organs:
Rarely: discomfort in the nose, dry nose, nosebleeds.
General disorders and disorders at the injection site:
Rarely: burning in the area of application.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor.
Interaction
Interaction
Phenylephrine (as well as other vasoconstrictors) is contraindicated in patients currently receiving MAO inhibitors or who have received them within the previous 2 weeks, as an interaction leading to increased blood pressure is possible.
Should not be taken concomitantly with tricyclic and tetracyclic antidepressants (eg, amitriptyline, mirtazapine): concomitant use with phenylephrine may increase the risk of developing vasoconstrictor effects. Should not be taken concomitantly with beta blockers and other antihypertensive drugs: phenylephrine may reduce the effectiveness of beta blockers and antihypertensive drugs. Accordingly, the risk of developing hypertension and other side effects from the cardiovascular system may increase.
If you are taking the above or other medications (including over-the-counter medications), consult your doctor before use.
Overdose
Overdose
An overdose of Vibrocil can cause sympathomimetic effects, for example, rapid heartbeat, premature contraction of the ventricles of the heart, headache in the back of the head, tremor, feeling of fatigue, increased blood pressure, emotional agitation, insomnia, pale skin.
The drug may also cause mild sedation, dizziness, stomach pain, nausea, and vomiting. Treatment: use of activated carbon, laxatives in young children from 1 year to 5 years inclusive (if possible); in adults and children over 6 years of age, take large amounts of fluid. There is no specific antidote. Increased
blood pressure caused by phenylephrine can be treated with alpha-blockers.
Further treatment with the drug should be carried out in accordance with clinical indications or based on the recommendations of the national poison control center.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
GSK Consumer Healthcare S.A., Switzerland
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | The drug should be kept out of reach of children, at a temperature not exceeding 30°C. |
| Manufacturer | GSC Consumer Healthcare S.A., Switzerland |
| Medication form | nasal drops |
| Brand | GSC Consumer Healthcare S.A. |
Related products
Buy Vibrocil, drops 15 ml with delivery to USA, UK, Europe and over 120 other countries.