No products in the cart.
Description
Vero-Fludarabine contains fludarabine phosphate, a fluorinated nucleotide analogue of the antiviral agent – vidarabine – 9-beta-D-arabinofuranosyladenine (ara-A), which is relatively resistant to deamination by adenosine deaminase.
In humans, fludarabine phosphate is rapidly dephosphorylated to 2-fluoro-ar-A, which, when taken up by cells, is then intracellularly phosphorylated to active triphosphate (2-fluoro-ar-AATP).
This metabolite inhibits RNA reductase, DNA polymerase (alpha-, delta- and ipsilon-), DNA primase and DNA ligase, which leads to disruption of DNA synthesis. In addition, RNA polymerase II is partially inhibited with a subsequent decrease in protein synthesis.
Pharmacokinetics
.No clear correlation was found between fludarabine pharmacokinetics and its therapeutic effect in cancer patients, and the incidence of neutropenia and hematocrit changes was dose-dependent. Fludarabine phosphate (2-fluoro-ara-AMP) is a water-soluble precursor of fludarabine (2-fluoro-ara-A) and is rapidly and completely dephosphorylated in the human body to 2-fluoro-ara-A nucleoside.
The binding to plasma proteins is insignificant. After a single infusion of a standard dose of the preparation for 30 minutes by patients with chronic lympholeucosis (CLL), the Cmax of 2-fluoroar-A in plasma was reached at the end of the infusion period (3.5-3.7 µM). After 5 injections of the drug a moderate increase in Cmax to 4.4-4.8 µM is detected by the end of the infusion.
Cumulation of 2-fluoro-ar-A after several cycles of therapy can be excluded. After the end of the infusion, there is a three-phase decrease in concentration with a T1/2 of the initial phase of about 5 minutes, an intermediate phase of 1-2 hours and a terminal phase of about 20 hours. 2-Fluoroura-A is excreted primarily by the kidneys (40 to 60% of the IV dose administered).
In persons with decreased renal function a decrease in total clearance is observed, indicating the need to reduce the dose. 2-Fluoroura-A is actively transported to leukemic cells, after which it is rephosphorylated to monophosphate and partially to di- and triphosphate.
Triphosphate (2-fluoroura-ATP) is the major intracellular metabolite and is the only known metabolite with cytotoxic activity.
The maximum level of 2-fluoroura-ATP in leukemic lymphocytes of patients with CLL is noted on average by 4 h and is characterized by considerable individual variability.
The concentration of 2-fluoroura-ATP in leukemic cells is significantly higher than in plasma, indicating its accumulation in tumor cells. T1/2 of 2-fluoroura-ATP from target cells averages 15 to 23 h.
Indications
Indications
Non-Hodgkin’s lymphomas of low malignancy (NHL NZ);
c-cell lympholeukemia (CLL).
Active ingredient
Active ingredient
Composition
Composition
Active ingredient:
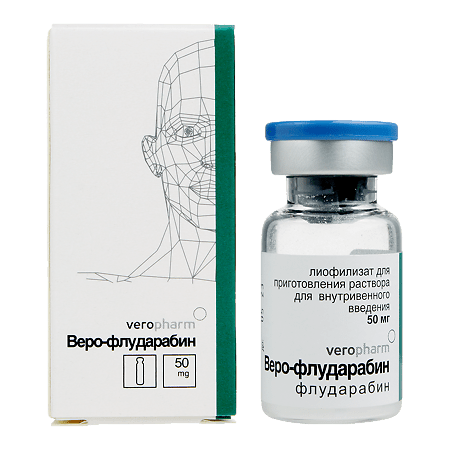
fludarabine phosphate 50 mg.
Excipients:
Mannitol;
Phosphate buffer solution
How to take, the dosage
How to take, the dosage
Only intravenous administration of fludarabine is allowed and accidental extravascular ingestion should be avoided.
Adults The recommended dose of fludarabine phosphate is 25 mg/m2 body surface area once daily by IV for 5 days every 28 days.
The required dose (calculated according to the patient’s body surface area) is drawn into a syringe.
For intravenous injection the above dose is diluted in 10 ml of 0.9% sodium chloride solution, or the required dose can be diluted in 100 ml of 0.9% sodium chloride solution and administered as a drop for 30 minutes.
There are no clear data on the optimal duration of treatment.
The course of treatment depends on the observed effect and tolerability of the drug.
The treatment with fludarabine is recommended until a therapeutic response is achieved (usually 6 cycles), after which the drug is stopped.
Interaction
Interaction
The use of fludarabine in combination with pentostatin (deoxycoformicin) to treat CLL has often been fatal due to high pulmonary toxicity.
Therapeutic efficacy of fludarabine may be reduced by adenosine reuptake inhibitors and dipyridamole. Pharmacokinetic interactions have been observed in patients with CLL and OML when treated with a combination of fludarabine and cytarabine.
When cytarabine and fludarabine are used together, higher intracellular peak concentrations and intracellular AUC of the cytarabine metabolite cytosine triphosphate have been shown.
Plasma concentrations of cytarabine and excretion of arabinosyl cytosine triphosphate were not altered. Fludarabine solution for IV administration should not be mixed with other drugs.
Contraindications
Contraindications
– reduced renal function with CK<30 ml/min;
– decompensated hemolytic anemia;
– pregnancy;
– period of breastfeeding;
– hypersensitivity to fludarabine or other components of the drug.
. The drug should be used with caution after careful assessment of the risk/benefit ratio in patients with weakened state, with significant reduction of bone marrow function (thrombocytopenia, anemia and/or granulocytopenia), immunodeficiency, acute viral, fungal or bacterial infection, liver failure (effectiveness and safety of fludarabine has not been studied), in children and patients over 75 years old (due to lack of clinical data on fludarabine use).
Side effects
Side effects
Blood organs: very common – neutropenia, thrombocytopenia and anemia; often – myelosuppression.
Immune system disorders: infrequent – autoimmune disorders (including autoimmune hemolytic anemia, thrombocytopenic purpura, vesicular disease, Evans syndrome, acquired hemophilia), allergic reactions.
In the digestive system: very frequently – nausea, vomiting, diarrhea; frequently – anorexia, stomatitis, mucositis; infrequently – gastro-intestinal bleeding, changes of liver and pancreatic enzymes activity.
Mechanical disorders: infrequent – as a result of lysis of tumor hyperuricemia, hyperphosphatemia, hypocalcemia, metabolic acidosis, hyperkalemia, hematuria, urate crystalluria may develop.
Nervous system disorders: often – peripheral neuropathy; infrequently – mental confusion; rarely – agitation, convulsions, coma.
An organ of vision: often – visual disturbances; rarely – optic neuritis, optic neuropathy and blindness.
Respiratory system disorders: very common – cough; infrequent – dyspnea, pulmonary fibrosis, pneumonitis.
Cardiovascular system: rarely – heart failure, arrhythmia.
Urinary system disorders: rarely – hemorrhagic cystitis.
Skin and skin appendages: often – skin rash; rarely – Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome).
Rare cases of increased growth of existing skin cancer and development of skin cancer during or after treatment with fludarabine have been reported. Other: very common – increased body temperature, increased fatigue, weakness, accession of secondary infections; common – chills, malaise, peripheral edema; rare – lymphoproliferative disorders (associated with Epstein-Barr virus).
In patients who received fludarabine before, after, or concomitantly with alkylating cytotoxic agents or radiotherapy, myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) has rarely been observed.
Overdose
Overdose
High doses of fludarabine may cause irreversible central nervous system changes, loss of vision, coma and death, as well as severe thrombocytopenia and neutropenia due to bone marrow suppression.
In case of threatening symptoms, the drug should be stopped immediately and supportive therapy should be given.
A specific antidote is not known.
Additional information
| Weight | 0.020 kg |
|---|---|
| Shelf life | 3 years. |
| Conditions of storage | At the temperature from 2 to 8 ° C in the light-protected place. |
| Manufacturer | Veropharm AO, Russia |
| Medication form | lyophilizate |
| Brand | Veropharm AO |
Related products
Buy Vero-Fludarabine, lyophilizate 50 mg with delivery to USA, UK, Europe and over 120 other countries.