No products in the cart.
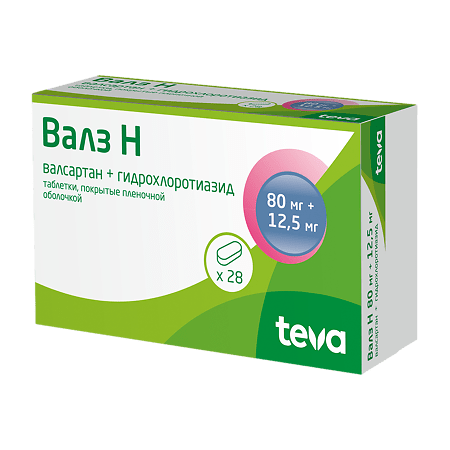
Valz N, 80 mg+12, 5 mg 28 pcs
€12.65 €10.54
Description
Pharmacotherapeutic group: combined hypotensive agent (angiotensin II receptor antagonist + diuretic).
The ATX code: C09DA03
Pharmacological properties
Pharmacodynamics
Valz N is a combination hypotensive drug consisting of an angiotensin II receptor antagonist (ARAII) and a thiazide diuretic.
Angiotensin II is the active hormone of the renin-angiotensin-aldosterone system (RAAS) and is formed from angiotensin I with the participation of angiotensin-converting enzyme (ACE). Angiotensin II binds to specific receptors located on cell membranes in various tissues. It has a wide range of physiological effects, including both direct and indirect involvement in blood pressure (BP) regulation. As an active vasoconstrictor, angiotensin II induces a direct pressor response. In addition, it stimulates the secretion of aldosterone and promotes the retention of sodium ions.
Valsartan is an active specific APAII. It selectively blocks AT1 subtype receptors, which are responsible for the vasopressor effect of angiotensin II. Increased serum concentrations of angiotensin II due to blockade of AT1 receptors by valsartan may result in stimulation of unblocked AT2 receptors, which partially counterbalances the vasopressor effects associated with AT1-receptor activation.
Valsartan has no agonist activity against AT1 receptors. The affinity of valsartan for AT1 receptors is approximately 20,000 times higher than for AT2 receptors.
Since valsartan does not inhibit ACE, which converts angiotensin I to angiotensin II and causes bradykinin degradation, the development of side effects associated with bradykinin accumulation is unlikely.
When comparing valsartan with an ACE inhibitor, the incidence of dry cough was significantly (p < 0.05) lower in patients receiving valsartan than in patients taking an ACE inhibitor (2.6% versus 7.9%, respectively).
Valsartan does not interact with or block other hormone receptors or ion channels involved in the regulation of cardiovascular function.
The site of action of thiazide diuretics is the distal convoluted renal tubules. When thiazide diuretics act on the highly sensitive receptors of the distal tubules of the cortical layer of the kidney, the reabsorption of sodium ions (Na+) and chlorine ions (Cl–) is suppressed. The suppression of the Na+ and Cl– co-transport system appears to be due to competition for the Cl– ion binding sites in this system. As a result, the excretion of sodium and chlorine ions increases to about the same extent. As a result of the diuretic action, there is a decrease in circulating blood volume, resulting in increased renin activity, aldosterone secretion, renal excretion of potassium and, consequently, decreased serum potassium content.
Pharmacokinetics
. Valsartan
Assimilation
After oral administration of valsartan, its maximum plasma concentration (Cmax) is reached within 2-4 hours. The average absolute bioavailability is 23%. When valsartan is taken with food, the area under the curve “concentration-time” (AUC) is reduced by 48%, although starting about 8 hours after taking the drug, its plasma concentrations, both when taken on an empty stomach and simultaneously with food, are the same. The decrease in AUC, however, is not accompanied by a clinically significant decrease in therapeutic effect, so valsartan can be taken regardless of the time of ingestion.
Distribution
The volume of distribution (Vd) of valsartan at equilibrium after intravenous (IV) administration was approximately 17 liters, indicating that it has no marked distribution in tissues. Valsartan is largely bound to serum proteins (94-97%), predominantly to albumin.
Metabolism
Valsartan is not significantly biotransformed (only about 20% of the ingested dose is excreted as metabolites). A pharmacologically inactive hydroxyl metabolite is detected in plasma at low concentrations (less than 10% of the AUC of valsartan).
Elevation
The pharmacokinetic curve of valsartan has a descending multiexponential character (the elimination half-life of the initial phase (T1/2α) less than 1 hour and the final phase (T1/2β) about 9 hours). Valsartan is excreted mainly unchanged through the intestine (about 83%) and the kidneys (about 13%). After IV administration, plasma clearance of valsartan is about 2 l/h, and its renal clearance is 0.62 l/h (about 30% of total clearance). The half-life (T1/2) is 6 hours.
In the range of doses studied, the kinetics of valsartan is linear. No changes in pharmacokinetic parameters were observed with its repeated administration. When valsartan is administered once daily, cumulation is insignificant. Plasma concentrations of valsartan do not differ between women and men.
Hydrochlorothiazide
Extraction
. After oral administration, absorption of hydrochlorothiazide is rapid, with a time to reach maximum concentration (Tmax) of about 2 hours.
In the therapeutic dose range, the average AUC increases in direct proportion to increasing the dose. Concomitant ingestion of hydrochlorothiazide with food may result in both increased and decreased systemic availability compared to fasting, but the magnitude of these effects is small and of little clinical significance. When oral administration the absolute bioavailability of hydrochlorothiazide is 70%.
Distribution
The pharmacokinetics of hydrochlorothiazide in the distribution and excretion phases is described in general by a biexponential downward curve. Apparent volume of distribution is 4-8 l/kg. Binding to plasma proteins (predominantly to albumin) is 40-70%. Hydrochlorothiazide also accumulates in erythrocytes at a concentration approximately three times higher than the plasma concentration.
Metabolism
Hydrochlorothiazide is excreted almost unchanged.
Elimation
The elimination half-life of the end phase is 6-15 hours. The kinetics of hydrochlorothiazide does not change with repeated administration; when the drug is administered once daily its accumulation is minimal. More than 95% of the absorbed dose is excreted unchanged by the kidneys.
Valsartan/hydrochlorothiazide
The systemic bioavailability of hydrochlorothiazide is reduced by approximately 30% when co-administered with valsartan. Concomitant use with hydrochlorothiazide has no significant effect on the pharmacokinetics of valsartan. This interaction has no effect on the efficacy of the combined use of valsartan and hydrochlorothiazide. Controlled clinical studies have demonstrated a significant antihypertensive effect of this combination that exceeded the effect of each component alone as well as the placebo effect.
Pharmacokinetics in selected patient groups
p> Elderly patients (> 65 years)
. Some elderly patients have higher systemic bioavailability of valsartan than younger patients; however, this has not been found to be clinically relevant.
Separate clinical data suggest that in elderly patients (both healthy and arterial hypertensive patients) the systemic clearance of hydrochlorothiazide is lower than in healthy young volunteers.
Patients with impaired renal function
There is no need to adjust the drug dose in patients with glomerular filtration rate (GFR) 30-70 ml/min/1.73 m2 body surface area.
There are currently no data on the use of the valsartan/hydrochlorothiazide combination in patients with severe renal impairment (GFR < 30 mL/min/1.73 m2 body surface area) and in patients receiving hemodialysis. Valsartan is not excreted by hemodialysis due to significant binding to plasma proteins. At the same time, hemodialysis allows efficient excretion of hydrochlorothiazide.
In the presence of renal failure, the mean peak plasma concentrations and AUC values of hydrochlorothiazide are increased and the excretion rate is decreased. In patients with mild to moderate renal impairment the half-life is almost doubled.
Patients with impaired liver function
The AUC of valsartan in patients with mild (Child-Pugh score 5-6) and moderate (Child-Pugh score 7-9) impaired liver function was 2 times greater than in healthy volunteers. There are currently no data on the use of the valsartan/hydrochlorothiazide combination in patients with severe (greater than 9 Child-Pugh points) hepatic impairment.
As hepatic impairment has no clinically significant effect on the pharmacokinetics of hydrochlorothiazide, no dose adjustment is required in patients with hepatic impairment.
The use of Valz H is contraindicated in patients with severe (greater than 9 points by Child-Pugh score) liver dysfunction, biliary obstruction (biliary cirrhosis and cholestasis).
Indications
Indications
Arterial hypertension (patients for whom combination therapy is indicated).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: combined antihypertensive drug (angiotensin II receptor antagonist + diuretic).
ATX code: C09DA03
Pharmacological properties
Pharmacodynamics
The drug Valz N is a combined antihypertensive drug, which includes an angiotensin II receptor antagonist (ARAII) and a thiazide diuretic.
Angiotensin II is an active hormone of the renin-angiotensin-aldosterone system (RAAS) and is formed from angiotensin I with the participation of angiotensin-converting enzyme (ACE). Angiotensin II binds to specific receptors located on cell membranes in various tissues. It has a wide range of physiological effects, including both direct and indirect participation in the regulation of blood pressure (BP). Being an active vasoconstrictor, angiotensin II causes a direct pressor response. In addition, it stimulates the secretion of aldosterone and promotes the retention of sodium ions.
Valsartan is an active specific APAII. It selectively blocks receptors of the AT1 subtype, which are responsible for the vasopressor effect of angiotensin II. An increase in serum angiotensin II concentration due to blockade of AT1 receptors by valsartan may lead to stimulation of unblocked AT2 receptors, which partly balances the vasopressor effects associated with activation of AT1 receptors.
Valsartan does not have agonist activity against AT1 receptors. The affinity of valsartan for receptors of the AT1 subtype is approximately 20,000 times higher than for receptors of the AT2 subtype.
Since valsartan does not inhibit ACE, which converts angiotensin I to angiotensin II and causes the destruction of bradykinin, the development of side effects associated with the accumulation of bradykinin is unlikely.
When comparing valsartan with an ACE inhibitor, the incidence of dry cough was significantly (p < 0.05) lower in patients receiving valsartan than in patients taking an ACE inhibitor (2.6% versus 7.9%, respectively).
Valsartan does not interact with or block receptors of other hormones or ion channels involved in the regulation of the functions of the cardiovascular system.
The point of action of thiazide diuretics is the distal convoluted renal tubules. When thiazide diuretics act on highly sensitive receptors of the distal tubules of the renal cortex, the reabsorption of sodium (Na+) and chlorine (Cl-) ions is suppressed. Suppression of the co-transport system of Na+ and Cl- apparently occurs due to competition for the binding sites of Cl- ions in this system. As a result, the excretion of sodium and chloride ions increases to approximately the same extent. As a result of the diuretic effect, a decrease in circulating blood volume is observed, as a result of which renin activity, aldosterone secretion, potassium excretion by the kidneys and, consequently, a decrease in the potassium content in the blood serum increase.
Pharmacokinetics
Valsartan
Suction
After taking valsartan orally, its maximum concentration (Cmax) in the blood plasma is achieved within 2-4 hours. The average absolute bioavailability is 23%. When taking valsartan with food, the area under the concentration-time curve (AUC) decreases by 48%, although, starting from approximately the 8th hour after taking the drug, its plasma concentrations are the same both when taken on an empty stomach and simultaneously with food. The decrease in AUC, however, is not accompanied by a clinically significant decrease in the therapeutic effect, so valsartan can be taken regardless of meal times.
Distribution
The volume of distribution (Vd) of valsartan during the steady-state period after intravenous (IV) administration was about 17 L, which indicates the absence of its pronounced distribution in tissues. Valsartan is highly bound to serum proteins (94-97%), mainly to albumin.
Metabolism
Valsartan does not undergo significant biotransformation (only about 20% of the dose taken orally is excreted in the form of metabolites). The pharmacologically inactive hydroxyl metabolite is found in blood plasma in low concentrations (less than 10% of the AUC of valsartan).
Removal
The pharmacokinetic curve of valsartan has a descending multi-exponential character (half-life of the initial phase (T1/2α) is less than 1 hour and the final phase (T1/2β) is about 9 hours). Valsartan is excreted mainly unchanged through the intestines (about 83%) and kidneys (about 13%). After intravenous administration, the plasma clearance of valsartan is about 2 l/h, and its renal clearance is 0.62 l/h (about 30% of the total clearance). The half-life (T1/2) is 6 hours.
In the range of doses studied, the kinetics of valsartan is linear. With its repeated use, no changes in pharmacokinetic parameters were observed. When taking valsartan once a day, the accumulation is insignificant. Plasma concentrations of valsartan do not differ between women and men.
Hydrochlorothiazide
Suction
After oral administration, absorption of hydrochlorothiazide occurs quickly, the time to reach maximum concentration (Tmax) is about 2 hours.
Over the therapeutic dose range, the mean AUC increases in direct proportion to the dose. Concomitant administration of hydrochlorothiazide with food may result in either increased or decreased systemic availability compared to administration on an empty stomach, but the magnitude of these effects is small and of little clinical significance. When taken orally, the absolute bioavailability of hydrochlorothiazide is 70%.
Distribution
The pharmacokinetics of hydrochlorothiazide during the distribution and elimination phases is generally described by a biexponential descending curve. The apparent volume of distribution is 4-8 l/kg. Binding to plasma proteins (mainly albumin) is 40-70%. Hydrochlorothiazide also accumulates in erythrocytes at concentrations approximately three times higher than plasma concentrations.
Metabolism
Hydrochlorothiazide is excreted virtually unchanged.
Removal
The half-life of the terminal phase is 6-15 hours. With repeated use, the kinetics of hydrochlorothiazide does not change; when the drug is prescribed once a day, its accumulation is minimal. More than 95% of the absorbed dose is excreted unchanged by the kidneys.
Valsartan/hydrochlorothiazide
When used together with valsartan, the systemic bioavailability of hydrochlorothiazide is reduced by approximately 30%. Co-administration with hydrochlorothiazide does not have a significant effect on the pharmacokinetics of valsartan. The observed interaction does not affect the effectiveness of the combined use of valsartan and hydrochlorothiazide. In controlled clinical studies, a pronounced antihypertensive effect of this combination was revealed, which exceeded the effect of each of the components separately, as well as the placebo effect.
Pharmacokinetics in selected patient groups
Elderly patients (> 65 years)
In some elderly patients, the systemic bioavailability of valsartan is higher than in younger patients, however, this has not been shown to be of clinical significance.
Some clinical data indicate that in elderly patients (both healthy and those suffering from arterial hypertension), the systemic clearance of hydrochlorothiazide is lower than in healthy young volunteers.
Patients with impaired renal function
No dose adjustment is required in patients with a glomerular filtration rate (GFR) of 30-70 ml/min/1.73 m2 body surface area.
There are currently no data on the use of the valsartan/hydrochlorothiazide combination in patients with severely impaired renal function (GFR < 30 ml/min/1.73 m2 body surface area) and in patients receiving hemodialysis. Valsartan is not eliminated by hemodialysis due to significant binding to plasma proteins. At the same time, hemodialysis allows hydrochlorothiazide to be effectively removed from the body.
In the presence of renal failure, the mean peak plasma concentrations and AUC value of hydrochlorothiazide increase and the rate of excretion decreases. In patients with mild to moderate renal impairment, the half-life is almost doubled.
Patients with liver dysfunction
The AUC of valsartan in patients with mild (5-6 points on the Child-Pugh scale) and moderate (7-9 points on the Child-Pugh scale) liver dysfunction was 2 times greater than in healthy volunteers. Currently, there are no data on the use of the combination of valsartan/hydrochlorothiazide in patients with severe (more than 9 points on the Child-Pugh scale) impaired liver function.
Since impaired liver function does not have a clinically significant effect on the pharmacokinetics of hydrochlorothiazide, no dosage adjustment is required in patients with impaired liver function.
The use of the drug Valz N is contraindicated in patients with severe (more than 9 points on the Child-Pugh scale) impaired liver function, obstruction of the biliary tract (biliary cirrhosis and cholestasis).
Special instructions
Special instructions
Changes in serum electrolytes
Valsartan
When used concomitantly with dietary supplements containing potassium, potassium-sparing diuretics, potassium-containing salt substitutes, or with other drugs that may cause an increase in potassium levels in the blood (for example, heparin), caution should be exercised and regular monitoring of potassium levels in the blood should be carried out.
Hydrochlorothiazide
Thiazide diuretics, due to their ability to reduce potassium and magnesium levels in the blood serum, should be used with caution in patients with conditions accompanied by electrolyte disturbances: salt-wasting nephropathy and prerenal (cardiogenic) renal dysfunction. If clinical manifestations of hypokalemia occur (muscle weakness, paresis, changes in ECG parameters), treatment with Valz N should be discontinued. Before starting to use the drug, it is necessary to correct hypokalemia and hypomagnesemia. All patients taking medications containing thiazide diuretics require regular monitoring of plasma electrolyte levels, especially potassium.
When using the drug Valz N, the ability of thiazide diuretics to cause hyponatremia and hypochloremic alkalosis, as well as aggravate existing hyponatremia, should be taken into account. Hyponatremia in these cases is rarely accompanied by neurological symptoms. Regular monitoring of sodium levels in blood plasma is necessary.
Sodium deficiency in the body and/or decreased blood volume
In patients receiving thiazide diuretics, including hydrochlorothiazide, it is necessary to monitor the appearance of clinical symptoms of sodium deficiency in the body and/or a decrease in blood volume.
In patients with severe sodium deficiency in the body and/or reduced blood volume, for example, receiving high doses of diuretics, in rare cases, after starting therapy with Valz N, a pronounced decrease in blood pressure may develop, accompanied by clinical manifestations. Before starting treatment with Valz N, it is necessary to adjust the sodium content in the body and/or replenish the blood volume, otherwise treatment must be started under strict medical supervision.
If a pronounced decrease in blood pressure develops, the patient should be laid down and, if necessary, given an intravenous infusion of 0.9% sodium chloride solution. After stabilization of blood pressure, treatment with Valz N can be continued.
Renal artery stenosis
In patients with unilateral or bilateral renal artery stenosis or stenosis of the artery of a single kidney, taking Valz N may be accompanied by an increase in the concentration of urea and creatinine in the blood serum, therefore, in such patients, Valz N should be used with caution.
Primary hyperaldosteronism
The drug is not effective for the treatment of arterial hypertension in patients with primary hyperaldosteronism, since activation of the RAAS is not observed in this category of patients.
Aortic and mitral stenosis, obstructive hypertrophic cardiomyopathy
As with other vasodilating agents, caution should be exercised when taking Valz N in patients with aortic or mitral stenosis and obstructive hypertrophic cardiomyopathy.
Renal dysfunction
Patients with mild or moderate renal impairment (GFR > 30 ml/min/1.73 m2 body surface area) do not require dose changes. In patients with impaired renal function when taking Valz N, periodic monitoring of the concentrations of potassium, creatinine and uric acid in the blood serum is required.
Kidney transplant
There is no experience with the use of the combination of valsartan/hydrochlorothiazide in patients with recent kidney transplantation.
Liver dysfunction
In patients with mild (5-6 points on the Child-Pugh scale) and moderate (7-9 points on the Child-Pugh scale) liver dysfunction without concomitant cholestasis, Valz N should be used with caution.
Chronic heart failure of functional class III-IV (NYHA classification), including after myocardial infarction.
In patients whose renal function depends on the state of the RAAS (for example, patients with chronic heart failure), therapy with ACE inhibitors and ARA II may be accompanied by oliguria and/or progressive azotemia, and in rare cases, acute renal failure.
Evaluation of patients with circulatory failure and patients who have had myocardial infarction should include a study of renal function.
Systemic lupus erythematosus
There are reports of exacerbation and worsening of connective tissue diseases (for example, systemic lupus erythematosus) with the use of thiazide diuretics, including hydrochlorothiazide.
Other metabolic disorders
Thiazide diuretics, including hydrochlorothiazide, may cause changes in glucose tolerance, as well as increases in serum concentrations of cholesterol, triglycerides and uric acid. In patients with diabetes mellitus, dose adjustment of hypoglycemic agents may be required.
Decreased uric acid clearance may lead to hyperuricemia and the development of gout in predisposed patients.
Thiazide diuretics reduce calcium excretion by the kidneys and may cause a slight increase in plasma calcium in the absence of concomitant disorders of calcium metabolism. Severe hypercalcemia during therapy with a thiazide diuretic (> 12 mg/dL) or not responding to drug discontinuation may indicate the presence of a concomitant disorder of calcium metabolism. In several patients with hypercalcemia and hypophosphatemia during long-term use of thiazide diuretics, pathological changes in the parathyroid glands were determined.
Before conducting a study of the parathyroid glands, it is necessary to stop taking thiazide diuretics.
Hypersensitivity reactions
The occurrence of hypersensitivity reactions during the use of hydrochlorothiazide was most often observed in patients with allergic reactions and a history of bronchial asthma.
During treatment with valsartan, there have been reports of the development of angioedema, accompanied by swelling of the larynx and vocal folds, leading to airway obstruction, and/or swelling of the face, lips, pharynx and/or tongue. Some patients in this group had previously experienced angioedema while taking other medications, including ACE inhibitors. If angioedema develops, the drug Valz N should be immediately discontinued, after which the resumption of the drug Valz N is prohibited.
Photosensitivity reactions
Photosensitivity reactions have been reported when taking thiazide diuretics. If photosensitivity reactions develop, it is recommended to stop taking the drug Valz N. If it is necessary to resume taking the diuretic, it is necessary to avoid exposure to ultraviolet radiation.
Choroidal effusion, acute myopia/secondary angle-closure glaucoma
Hydrochlorothiazide can cause an idiosyncratic reaction leading to the development of acute myopia and an acute attack of secondary angle-closure glaucoma. Symptoms include: sudden loss of vision or eye pain, usually occurring within hours to weeks of starting hydrochlorothiazide therapy. If left untreated, acute angle-closure glaucoma can lead to irreversible vision loss. If symptoms appear, you should stop taking hydrochlorothiazide as soon as possible. If intraocular pressure remains uncontrolled, emergency medical treatment or surgery may be required. Risk factors for the development of acute angle-closure glaucoma are: a history of an allergic reaction to sulfonamides or penicillin.
Non-melanoma skin cancer
Two pharmacoepidemiological studies using data from the Danish National Cancer Registry demonstrated an association between hydrochlorothiazide use and an increased risk of non-melanoma skin cancer (NMSC) basal cell carcinoma and squamous cell carcinoma. The risk of developing NMSC increased with increasing total (cumulative) dose of hydrochlorothiazide. A possible mechanism for the development of NMSC is the photosensitizing effect of hydrochlorothiazide.
Patients taking hydrochlorothiazide as monotherapy or in combination with other drugs should be aware of the risk of developing NMSC. In such patients, it is recommended that the skin be examined to identify any new suspicious lesions, as well as changes in existing skin lesions.
Any suspicious skin changes should be reported to your doctor immediately. Suspicious areas of skin should be examined by a specialist. To clarify the diagnosis, histological examination of skin biopsies may be required.
To minimize the risk of developing NMSC, patients should be advised to follow preventive measures, such as limiting exposure to sunlight and UV rays, and using appropriate protective equipment.
In patients with a history of non-melanoma skin cancer, it is recommended to reconsider the use of hydrochlorothiazide.
Doping control results
Hydrochlorothiazide may give a positive result during doping control.
Impact on the ability to drive vehicles and machinery
Since adverse reactions such as dizziness or fainting may develop during drug therapy, patients taking the drug Valz N should be careful when driving vehicles and engaging in other potentially hazardous activities.
Active ingredient
Active ingredient
Valsartan, Hydrochlorothiazide
Composition
Composition
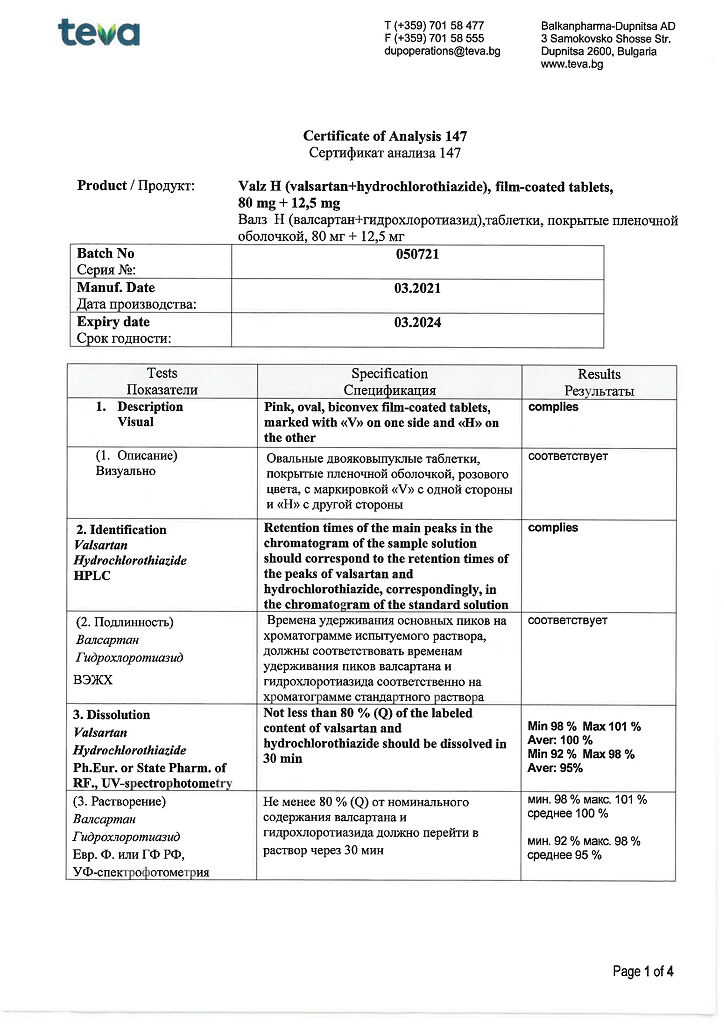
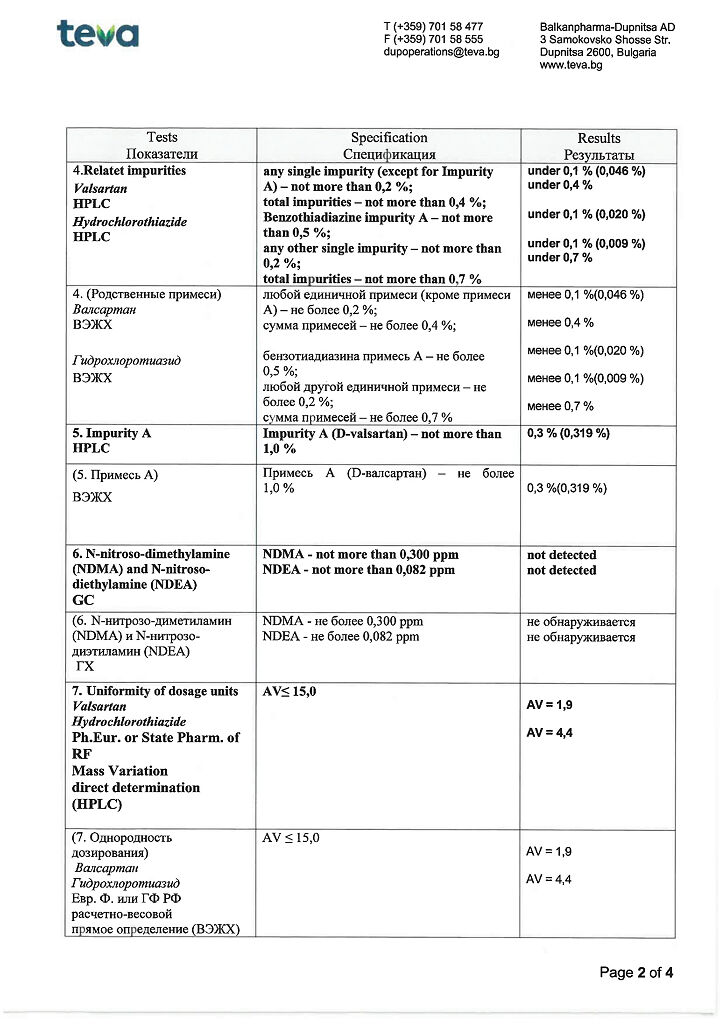
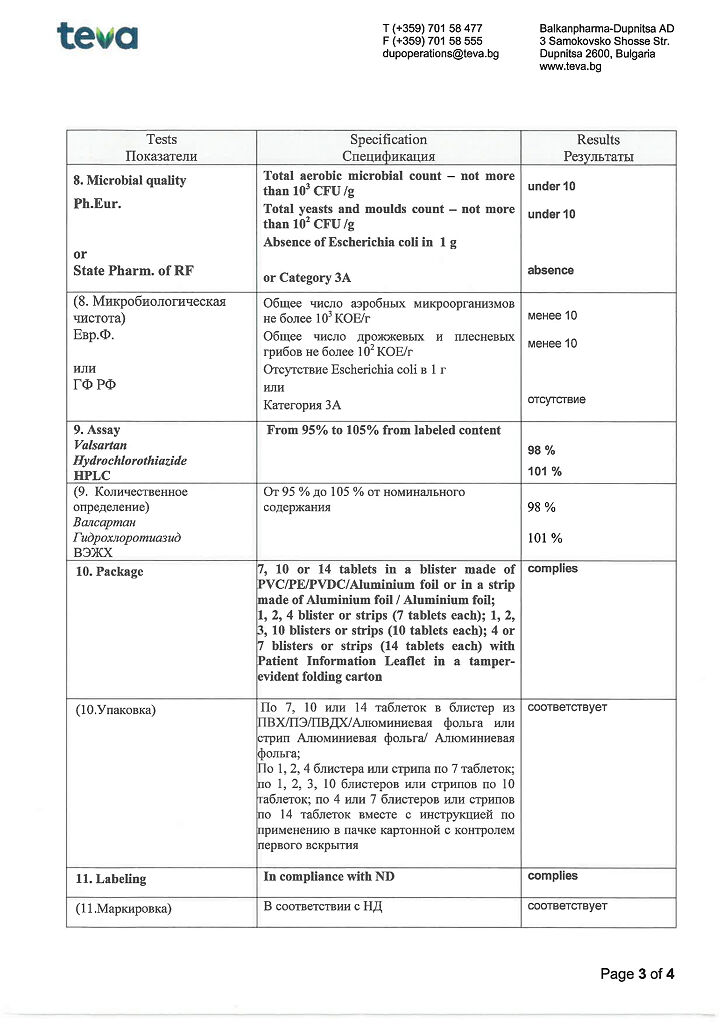
for dosage 80 mg + 12.5 mg
active ingredients: valsartan 80.00 mg, hydrochlorothiazide 12.50 mg;
excipients: microcrystalline cellulose pH 102 – 36.00 mg, lactose monohydrate – 29.72 mg, croscarmellose sodium – 10.80 mg, povidone K29/32 – 7.20 mg, talc – 1.80 mg, magnesium stearate – 1.26 mg, colloidal silicon dioxide – 0.72 mg;
film shell: Opadry II 85G34642 Pink 7.20 mg (polyvinyl alcohol – 3.17 mg, talc – 1.44 mg, titanium dioxide – 1.39 mg, macrogol 3350 – 0.89 mg, lecithin – 0.25 mg, iron dye red oxide – 0.03 mg, iron dye yellow oxide – 0.03 mg, iron dye black oxide – 0.0006 mg).
Pregnancy
Pregnancy
Pregnancy
Summary of Risks
As with any other drug that affects the RAAS, the use of Valz N during pregnancy is contraindicated. Given the mechanism of action of APAII, a risk to the fetus cannot be excluded. It is known that the use of ACE inhibitors (a class of specific drugs acting on the RAAS) in the second and third trimesters of pregnancy leads to damage and death of the fetus. According to a retrospective analysis, the use of ACE inhibitors in the first trimester of pregnancy is associated with a potential risk of fetal malformations.
Hydrochlorothiazide crosses the placenta. With unintentional use of valsartan during pregnancy, cases of spontaneous abortion, oligohydramnios and renal dysfunction in newborns have been described.
In utero fetal exposure to thiazide diuretics, including hydrochlorothiazide, is associated with the development of jaundice or thrombocytopenia in the fetus or neonate and may also be associated with other adverse reactions reported in adults. If pregnancy is diagnosed during treatment with Valz N, the drug should be discontinued as early as possible.
Clinical aspects
Maternal and/or embryofetal risk associated with the disease
Arterial hypertension during pregnancy increases the risk of developing preeclampsia, gestational diabetes mellitus, premature birth, and complications during childbirth (for example, the need for cesarean section, postpartum hemorrhage). Arterial hypertension increases the risk of intrauterine growth retardation and intrauterine fetal death.
Fetal/newborn risk
Oligohydramnios in pregnant women taking drugs that affect the RAAS in the second and third trimester can lead to deterioration of fetal renal function, resulting in anuria and renal failure, fetal lung hypoplasia, fetal skeletal deformation, including hypoplasia of the skull bones, arterial hypotension and fetal death.
If ARBs are inadvertently taken during pregnancy, the need for appropriate fetal monitoring should be considered.
Newborns whose mothers received ARA therapy should be carefully monitored for the development of arterial hypotension.
Valsartan
When studying embryofetal development in mice, rabbits and rats, fetotoxicity was observed, which was associated with maternal toxicity in rats using valsartan at a daily dose of 600 mg/kg per day, which is approximately 6 times the maximum recommended daily dose for a patient weighing 60 kg), and rabbits when using valsartan at a daily dose of 10 mg/kg, which is approximately 0.6 times higher the maximum recommended daily dose for a person based on mg/kg body weight (calculation assumes a daily dose of 320 mg orally for a patient with a human body weight of 60 kg). No maternal toxicity or fetotoxicity was observed when used in mice at daily doses up to 600 mg/kg, which is approximately 9 times the maximum recommended daily dose for humans based on mg/kg body weight (calculation assumes a daily dose of 320 mg orally for a patient weighing 60 kg).
Hydrochlorothiazide
There was no teratogenicity or effect of hydrochlorothiazide on fertility or fertilization in a study of 3 animal species that received hydrochlorothiazide orally. In rats, no dose-dependent fetotoxicity was observed when administered orally at doses of 0, 100, 300 and 1000 mg/kg. The delay in body weight gain in dairy rat pups was due to high doses and the diuretic effect of hydrochlorothiazide and the corresponding effect on milk production.
Breast-feeding
It is not known whether valsartan passes into breast milk. Preclinical studies have shown that valsartan is excreted in the milk of lactating rats. Hydrochlorothiazide is excreted in breast milk. The use of the drug during breastfeeding is contraindicated.
Patients and patients with preserved reproductive potential
Like any other drug that has a direct effect on the RAAS, Valz N should not be used in women planning pregnancy. When choosing any drug that affects the RAAS, the doctor should inform the patient with preserved reproductive potential about the possible risk of using the drug during pregnancy.
Fertility
There are no data on the effects of valsartan or hydrochlorothiazide on fertility in humans. In preclinical studies, there was no effect of valsartan or hydrochlorothiazide on fertility in rats.
Contraindications
Contraindications
Hypersensitivity to valsartan, hydrochlorothiazide and other sulfonamide derivatives, or any other component of the drug;
pregnancy and pregnancy planning, breastfeeding period;
severe liver dysfunction (more than 9 points on the Child-Pugh scale), biliary cirrhosis and cholestasis;
anuria, severe renal dysfunction (GFR < 30 ml/min/1.73 m2 body surface area);
children under 18 years of age (the effectiveness and safety of the drug in this category of patients have not yet been established);
refractory hypokalemia, hyponatremia, hypercalcemia and symptomatic hyperuricemia;
simultaneous use with aliskiren and drugs containing aliskiren in patients with diabetes mellitus and/or moderate or severe renal impairment (GFR < 60 ml/min/1.73 m2 body surface area);
simultaneous use with ACE inhibitors in patients with diabetic nephropathy;
lactose intolerance, lactase deficiency, glucose-galactose malabsorption (the drug contains lactose monohydrate).
With caution
Concomitant use with potassium-sparing food supplements, potassium-sparing diuretics, potassium-containing salt substitutes, as well as other drugs that can increase potassium levels.
Unilateral or bilateral renal artery stenosis or stenosis of the artery of a single kidney.
Conditions accompanied by disturbances of water and electrolyte balance: nephropathy, accompanied by loss of salts, and prerenal (cardiogenic) renal dysfunction; hypokalemia, hyponatremia, hypercalcemia.
Severe sodium deficiency and/or decreased circulating blood volume (CBV) (for example, in patients receiving high doses of diuretics).
Moderate liver dysfunction (class B according to the Child-Pugh classification).
Chronic heart failure (CHF) functional class III-IV according to the NYHA classification, mitral or aortic stenosis, hypertrophic obstructive cardiomyopathy.
Systemic lupus erythematosus.
Primary hyperaldosteronism.
Diabetes mellitus, hyperuricemia.
Hypercholesterolemia and hypertriglyceridemia.
Obstructive diseases of the biliary tract and cholestasis.
History of allergic reactions to penicillin. Condition after kidney transplantation.
Concomitant use of drugs that can cause polymorphic ventricular tachycardia of the “pirouette” type.
Gout.
History of non-melanoma skin cancer.
Concomitant use of drugs that can cause hypokalemia and lithium preparations.
Side Effects
Side Effects
Adverse reactions (ARs) according to clinical and post-marketing studies were more common when using valsartan and hydrochlorothiazide compared to placebo.
During therapy with Valz N, adverse reactions may occur, which were observed both when using valsartan and hydrochlorothiazide separately, and were not identified during clinical trials of Valz N.
To assess the frequency of ADRs, the following criteria were used (according to the World Health Organization (WHO) classification): very common (>10%); often (>1% and <10%); uncommon (>0.1% and <1%); rare (>0.01% and <0.1%); very rare (<0.01%), frequency unknown (the frequency of development cannot be determined from the available data).
System-organ class
Adverse reactions
Valsartan+
Hydrochlorothiazide
Valsartan
Hydrochlorothiazide
Benign, malignant and unspecified neoplasms (including cysts and polyps)
non-melanoma skin cancer (basal cell carcinoma of the skin and squamous cell carcinoma of the skin)
–
–
frequency unknown
Blood and lymphatic system disorders
thrombocytopenia, sometimes in combination with purpura
–
frequency unknown
rarely
agranulocytosis
–
–
very rarely
suppression of bone marrow hematopoiesis
–
–
very rarely
hemolytic anemia
–
–
very rarely
leukopenia
–
–
very rarely
decrease in hemoglobin
–
frequency unknown
–
decrease in hematocrit
–
frequency unknown
–
aplastic anemia
–
–
frequency unknown
Immune system disorders
hypersensitivity reactions
–
–
very rarely
hypersensitivity phenomena/allergic reactions, including serum sickness
–
frequency unknown
–
Metabolic and nutritional disorders
increased concentration of lipids in blood plasma (especially against the background of high doses of hydrochlorothiazide)
–
–
very often
hypomagnesemia
–
–
often
hyponatremia
–
frequency unknown
–
hyperuricemia
–
–
often
hypercalcemia
–
–
rarely
hyperglycemia
–
–
rarely
glucosuria
–
–
rarely
decrease in bcc
infrequently
–
–
worsening diabetes mellitus
–
–
rarely
hypochloremic alkalosis
–
–
very rarely
increased serum potassium levels
–
frequency unknown
–
Mental disorders
sleep disorders
–
–
rarely
depression
–
–
rarely
Nervous system disorders
headache
often
–
rarely
paresthesia
infrequently
–
rarely
dizziness
very rarely
rarely
fainting
frequency unknown
–
–
Visual disorders
decreased visual acuity
infrequently
–
–
blurred vision (especially in the first few weeks of treatment)
–
–
rarely
acute attack of angle-closure glaucoma
–
–
frequency unknown
choroidal effusion
–
–
frequency unknown
Hearing and labyrinth disorders
tinnitus
infrequently
–
–
vertigo
–
infrequently
–
Heart disorders
arrhythmias
–
–
rarely
Vascular disorders
orthostatic hypotension (may be worsened by alcohol, sedatives, or painkillers)
–
–
often
pronounced decrease in blood pressure
infrequently
–
–
peripheral edema
infrequently
–
–
vasculitis
–
frequency unknown
–
Respiratory, thoracic and mediastinal disorders
cough
infrequently
–
–
respiratory distress syndrome, including pulmonary edema and pneumonitis
–
–
very rarely
non-cardiogenic pulmonary edema
frequency unknown
–
–
Gastrointestinal disorders
loss of appetite
–
–
often
moderate nausea
–
–
often
nausea
infrequently
–
–
vomit
–
–
often
stomach pain
–
infrequently
–
abdominal discomfort
–
–
rarely
constipation
–
–
rarely
diarrhea
very rarely
–
rarely
pancreatitis
–
–
very rarely
Disorders of the liver and biliary tract
intrahepatic cholestasis or jaundice
–
–
rarely
increased activity of liver enzymes
–
frequency unknown
–
Skin and subcutaneous tissue disorders
hives and other types of skin rashes
–
–
often
photosensitivity
–
–
rarely
necrotizing vasculitis and toxic epidermal necrolysis
–
–
very rarely
lupus-like reactions, exacerbation of skin manifestations of systemic lupus erythematosus
–
–
very rarely
angioedema
–
frequency unknown
–
skin rash
–
frequency unknown
–
itchy skin
–
frequency unknown
–
bullous dermatitis
–
frequency unknown
–
erythema multiforme
–
–
frequency unknown
Musculoskeletal and connective tissue disorders
myalgia
infrequently
–
–
arthralgia
very rarely
–
–
muscle spasms
–
–
frequency unknown
Renal and urinary tract disorders
renal failure
–
frequency unknown
–
acute renal failure
–
–
frequency unknown
renal dysfunction
frequency unknown
–
frequency unknown
Disorders of the genital organs and breast
impotence
–
–
often
General and administration site disorders
increased fatigue
infrequently
–
–
hyperthermia
–
–
frequency unknown
asthenia
–
–
frequency unknown
Laboratory and instrumental data
increased concentration of uric acid in blood serum
frequency unknown
–
–
increased serum bilirubin
frequency unknown
–
–
increase in serum creatinine
frequency unknown
–
–
hypokalemia
frequency unknown
–
–
hyponatremia
frequency unknown
–
–
increase in serum urea nitrogen concentration
frequency unknown
–
–
neutropenia
frequency unknown
–
–
The following adverse events were observed in patients with arterial hypertension during clinical trials of the drug Valz N without an obvious connection with the drug: abdominal pain, upper abdominal pain, anxiety, arthritis, asthenia, back pain, bronchitis (including acute), chest pain, postural dizziness, dyspepsia, shortness of breath, dry oral mucosa, nosebleeds, erectile dysfunction, gastroenteritis, headache pain, increased sweating, hypoesthesia, flu-like condition, insomnia, sprain, muscle spasms, muscle hypertonicity, nasal congestion, nasopharyngitis, nausea, neck pain, peripheral edema, otitis media, pain in the extremities, rapid heartbeat, pain in the larynx and pharynx, pyrexia, pollakiuria, hyperthermia, sinusitis, drowsiness, upper respiratory tract infections, urinary tract infections, vertigo, viral infections, visual impairment.
The following adverse events were observed during clinical trials of valsartan in patients with arterial hypertension, regardless of their causal relationship with the study drug: arthralgia, asthenia, back pain, diarrhea, dizziness, headache, insomnia, decreased libido, nausea, edema, pharyngitis, rhinitis, sinusitis, upper respiratory tract infections, viral infections.
Interaction
Interaction
Common drug interactions for valsartan and hydrochlorothiazide
Medicines that should be avoided when combined with:
Lithium preparations
With the simultaneous use of lithium preparations with ACE and ARA II inhibitors or thiazide diuretics, a reversible increase in the concentration of lithium in the blood plasma and an associated increase in toxic manifestations were observed. The risk of toxicities associated with the use of lithium preparations may be further increased when used concomitantly with Valz N, since the renal clearance of lithium preparations is reduced by the influence of thiazide diuretics.
Medicines that require caution when used together:
Antihypertensive drugs
The antihypertensive effect may be enhanced when used together with other drugs that lower blood pressure (ACE inhibitors, beta-blockers, slow calcium channel blockers, guanethidine, methyldopa, vasodilators, direct renin inhibitors, ARAII).
Pressor amines
The effect of pressor amines (norepinephrine, epinephrine) may be weakened, without requiring cessation of joint use.
Nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase-2 (COX-2) inhibitors
NSAIDs, when taken concomitantly, can weaken the antihypertensive effect of both angiotensin II receptor antagonists and hydrochlorothiazide. The simultaneous use of Valz N and NSAIDs can lead to impaired renal function and an increase in potassium levels in the blood serum. If it is necessary to use the drug Valz N and NSAIDs together, before starting treatment, it is necessary to assess renal function and correct water and electrolyte disturbances.
Drug interactions for valsartan
Medicines that should be avoided when combined with:
Simultaneous use of potassium-sparing diuretics, dietary supplements containing potassium; potassium-containing substitutes for table salt; other drugs that increase potassium levels in the blood serum (for example, heparin) require precautions (including frequent determination of potassium levels in the blood).
Double blockade of the RAAS
When treated with drugs that affect the RAAS, especially when they are combined, a marked decrease in blood pressure, syncope, stroke, hyperkalemia and impaired renal function (including acute renal failure) have been reported in sensitive patients.
Caution is required when combining ARBs, including valsartan, with other drugs that block the RAAS, such as ACE inhibitors or aliskiren.
Concomitant use of ARBs, including valsartan, with drugs containing aliskiren is contraindicated in patients with diabetes mellitus and/or with moderate or severe renal impairment (GFR < 60 ml/min/1.73 m2 body surface area) and is not recommended in other patients.
Concomitant use of ARBs with ACE inhibitors is contraindicated in patients with diabetic nephropathy and is not recommended in other patients.
Transport proteins
According to the results of an in vitro study on liver cultures, valsartan is a substrate for the transporter proteins OATP1B1 and MRP2. Co-administration of valsartan with inhibitors of the OATP1B1 transport protein (rifampicin, cyclosporine) and with an inhibitor of the MRP2 transport protein (ritonavir) may increase the systemic exposure of valsartan (Cmax and AUC).
No drug interactions:
There were no clinically significant interactions observed during monotherapy with valsartan while using the following drugs: cimetidine, warfarin, furosemide, digoxin, atenolol, indomethacin, hydrochlorothiazide, amlodipine, glibenclamide.
Drug interactions for hydrochlorothiazide
Lithium
When used simultaneously with ACE inhibitors and diuretics, cases of reversible increases in plasma lithium concentrations and its toxic effects have been reported. The combined use of hydrochlorothiazide with valsartan and lithium preparations has not been studied. Therefore, when using hydrochlorothiazide and drugs containing lithium simultaneously, it is recommended to monitor the concentration of lithium in the blood.
Other antihypertensive drugs
Thiazide diuretics enhance the antihypertensive effect of other antihypertensive drugs (including guanethidine, methyldopa, beta-blockers, vasodilators, slow calcium channel blockers, ACE inhibitors, angiotensin receptor antagonists, renin inhibitors).
Curare-like muscle relaxants
Thiazide diuretics, including hydrochlorothiazide, potentiate the action of non-depolarizing muscle relaxants.
Medicines that affect potassium levels in the blood
The risk of hypokalemia caused by diuretics may be increased by concomitant use of glucocorticosteroids (GCS), laxatives, adrenocorticotropic hormone (ACTH), amphotericin B, carbenoxolone, penicillin, acetylsalicylic acid or its derivatives and antiarrhythmic drugs.
Medicines that affect sodium levels in the blood
The hyponatremic effect caused by diuretics may be enhanced when used simultaneously with antidepressants, antipsychotics, anticonvulsants (carbamazepine), etc. Caution should be exercised during long-term co-administration of hydrochlorothiazide with the above drugs.
Medicines that can provoke polymorphic ventricular tachycardia of the “pirouette” type:
Class IA antiarrhythmic drugs (quinidine, hydroquinidine, disopyramide).
Class III antiarrhythmic drugs (amiodarone, dofetilide, ibutilide), sotalol.
Some antipsychotics (thioridazine, chlorpromazine, levomepromazine, trifluoperazine, cyamemazine, sulpiride, sultopride, amisulpride, tiapride, pioside, haloperidol, droperidol).
Other drugs (bepridil, cisapride, difemanil, erythromycin IV, halofantrine, ketanserin, mizolastine, pentamidine, sparfloxacin, terfenadine, vincamine IV).
Due to the risk of developing hypokalemia, hydrochlorothiazide should be used with caution concomitantly with drugs that can cause polymorphic ventricular tachycardia of the torsade de pointes type.
Hypoglycemic agents
Thiazide diuretics may alter glucose tolerance, which may require dosage adjustments of insulin and oral hypoglycemic agents.
Metformin should be used with caution due to the risk of developing lactic acidosis caused by possible renal impairment associated with hydrochlorothiazide.
Beta blockers and diazoxide
Concomitant use of thiazide diuretics, including hydrochlorothiazide, with beta-blockers may increase the risk of hyperglycemia. Thiazide diuretics, including hydrochlorothiazide, may enhance the hyperglycemic effect of diazoxide.
Medicines used to treat gout (probenecid, sulfinpyrazone and allopurinol)
Dosage adjustment of uricosuric agents (probenecid or sulfinpyrazone) may be necessary because hydrochlorothiazide may increase serum uric acid concentrations. Concomitant use of thiazide diuretics, including hydrochlorothiazide, may increase the incidence of hypersensitivity reactions to allopurinol.
Cardiac glycosides
Hypokalemia and hypomagnesemia (adverse effects of thiazide diuretics) may contribute to the development of cardiac arrhythmias in patients receiving cardiac glycosides.
NSAIDs
The simultaneous use of NSAIDs and hydrochlorothiazide may lead to a decrease in the diuretic and antihypertensive effects of the latter. Concomitant hypovolemia can provoke the development of acute renal failure.
N- and m-anticholinergics
H- and m-anticholinergic agents (including atropine, biperiden) can increase the bioavailability of hydrochlorothiazide, which is associated with a decrease in gastrointestinal motility and the rate of gastric emptying. Accordingly, gastrointestinal motility stimulants (cisapride) may reduce the bioavailability of hydrochlorothiazide.
Anion exchange resins
The absorption of hydrochlorothiazide is impaired in the presence of cholestyramine and colestipol. Hydrochlorothiazide should be taken either 4 hours before or 4-6 hours after taking these compounds.
Vitamin D and calcium salts
Concomitant use of hydrochlorothiazide with vitamin D or calcium supplements can lead to hypercalcemia due to increased calcium reabsorption.
Cyclosporine
With the simultaneous use of hydrochlorothiazide and cyclosporine, the risk of developing hyperuricemia and exacerbation of gout increases.
Methyldopa
Cases of hemolytic anemia have been reported with the simultaneous administration of hydrochlorothiazide and methyldopa.
Other types of interaction
Co-administration of thiazide diuretics, including hydrochlorothiazide, may lead to an increased risk of side effects from amantadine; reducing the excretion by the kidneys of drugs that have a cytotoxic effect (for example, cyclophosphamide, methotrexate), to potentiate their myelosuppressive effect.
Ethanol, barbiturates and narcotic drugs
Their combined use with hydrochlorothiazide may potentiate the development of orthostatic hypotension.
Iodinated contrast agents
When using iodine contrast agents in high doses against the background of a reduced volume of blood volume due to the use of diuretics, there is an increased risk of developing acute renal failure. Before you start taking the drug Valz N, you should replenish your blood volume.
Overdose
Overdose
Symptoms: in case of an overdose of valsartan, severe hypotension can be expected to develop, accompanied by depression of consciousness, vascular collapse and/or shock with a fatal outcome. In case of an overdose of hydrochlorothiazide, the following symptoms may appear: nausea, drowsiness, hypovolemia, cardiac arrhythmias and muscle spasms caused by water and electrolyte imbalance.
Treatment: symptomatic, the nature of which depends on the time elapsed since taking the drug and the severity of the symptoms. In case of early detection of a drug overdose, it is recommended to induce vomiting in the patient. In the event of a pronounced decrease in blood pressure, an intravenous infusion of 0.9% sodium chloride solution is recommended, accompanied by regular monitoring of the activity of the heart and respiratory system, blood volume and the amount of urine excreted. For the period of time required for therapy, the patient should be laid down with his legs elevated.
Valsartan is not eliminated by hemodialysis due to significant binding to plasma proteins. At the same time, hemodialysis can effectively remove hydrochlorothiazide from the body.
Storage conditions
Storage conditions
Store at a temperature not exceeding 30 °C.
Keep out of the reach of children!
Shelf life
Shelf life
3 years. Do not use after expiration date.
Manufacturer
Manufacturer
Balkanpharma-Troyan AD, Bulgaria
Additional information
| Shelf life | 3 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | Store at a temperature not exceeding 30 °C. Keep out of reach of children! |
| Manufacturer | Balkanpharma – Troyan AD, Bulgaria |
| Medication form | pills |
| Brand | Balkanpharma – Troyan AD |
Other forms…
Related products
Buy Valz N, 80 mg+12, 5 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.