No products in the cart.
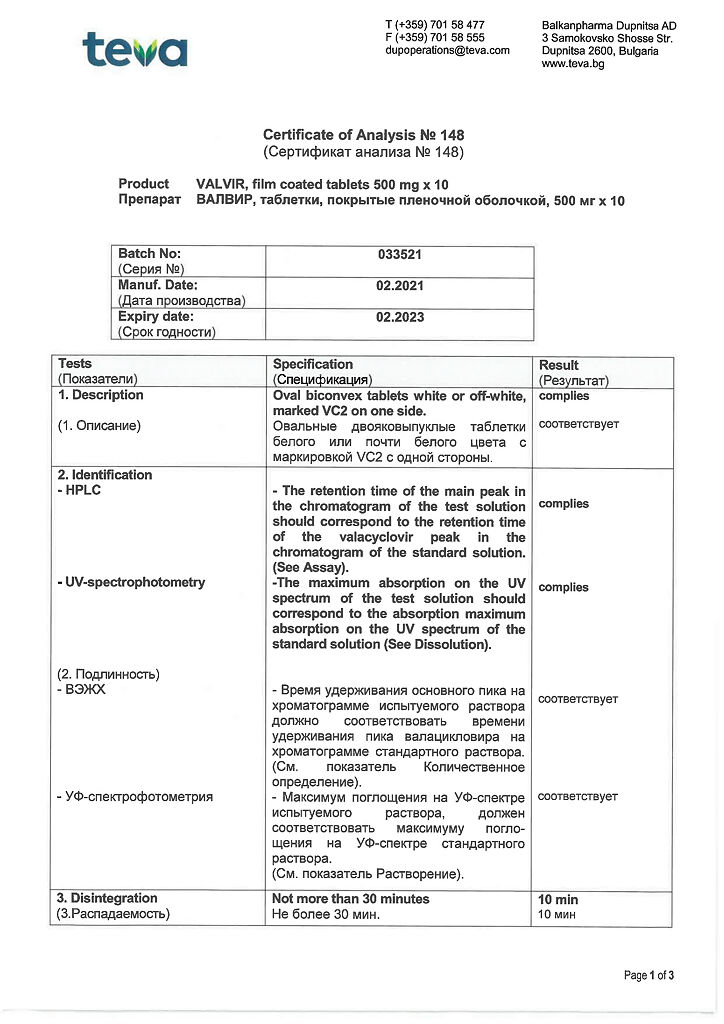
Description
Pharmacodynamics
Valvir is an antiviral drug. In the human body valacyclovir is quickly and completely converted into acyclovir and L-valine under the influence of valacyclovir hydrolase. In vitro acyclovir has specific inhibitory activity against Herpes simplex viruses types 1 and 2, Varicella zoster and Epstein-Barr, cytomegalovirus (CMV) and human herpes virus type 6.
Aciclovir inhibits the synthesis of viral deoxyribonucleic acid (DNA) immediately after phosphorylation and conversion into the active form acyclovir triphosphate. The first stage of phosphorylation occurs with the participation of virus-specific enzymes.
For Herpes simplex, Varicella zoster and Epstein-Barr viruses this enzyme is viral thymidine kinase, which is present in virus-infected cells. Partial phosphorylation selectivity is retained in CMV and is mediated through the phosphotransferase gene product UL 97. Activation of acyclovir by a specific viral enzyme explains much of its selectivity.
The process of acyclovir phosphorylation (conversion from mono- to triphosphate) is completed by cellular kinases. Acyclovir triphosphate competitively inhibits viral DNA polymerase and, being a nucleoside analog, is incorporated into viral DNA, which leads to obligate (complete) chain breaking, termination of DNA synthesis and, consequently, to blocking of virus replication.
In immunocompromised patients, Herpes simplex and Varicella zoster viruses with hypersensitivity to Valacyclovir are extremely rare (less than 0.1%), but can sometimes be found in patients with severe immune disorders, such as those with bone marrow transplants, those receiving chemotherapy for malignancies and those infected with human immunodeficiency virus (HIV).
Resistance is caused by a thymidine kinase deficiency of the virus, which leads to excessive spread of the virus in the host. Sometimes decreased sensitivity to acyclovir is caused by the emergence of virus strains with disrupted viral thymidine kinase or DNA polymerase structure. Virulence of these varieties of the virus is similar to that of its wild strain.
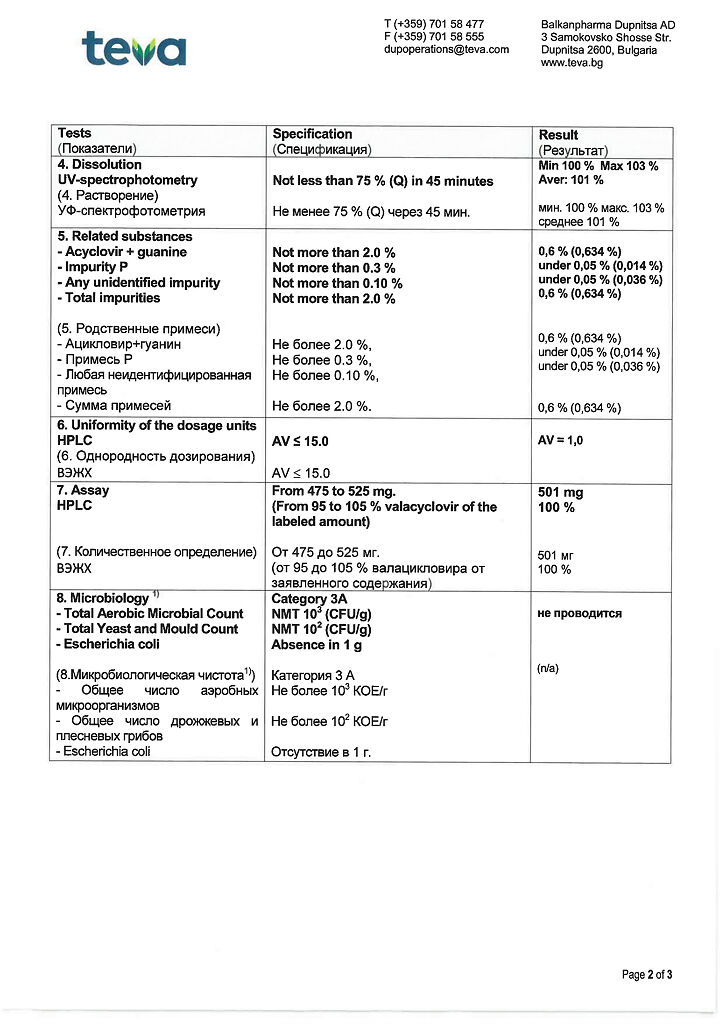
Pharmacokinetics
Valacyclovir and acyclovir have similar pharmacokinetic parameters after oral administration.
Intestation
After oral administration, valacyclovir is well absorbed from the gastrointestinal tract (GIT) and is rapidly and almost completely converted to acyclovir and L-valine. This conversion is catalyzed by the enzyme valacyclovirhydrolase isolated from human liver.
After a single dose of 0.25-2 g of valacyclovir, the maximum plasma concentration (Cmax) of acyclovir in healthy volunteers with normal renal function averages 10-37 µmol/L (2.2-8.3 µg/mL), and the median time to reach this concentration is 1 -2 hours.
The bioavailability of acyclovir is 54% when valacyclovir is taken at a dose of 1 g or more and is not dependent on food intake.
The Cmax of valacyclovir in plasma is only 4% of the acyclovir concentration and is reached on average 30-100 min after taking the drug; after 3 h the Cmax level remains the same or decreases.
Distribution
The degree of binding of acyclovir to plasma proteins is very low – about 15%. Acyclovir is rapidly distributed throughout the body tissues, especially in the liver, kidneys, muscles and lungs. It also penetrates into the vaginal secretion, cerebrospinal fluid and fluid of herpetic vesicles.
Elimation
In patients with normal renal function, the half-life (T1/2) of acyclovir after a single dose and repeated administration is approximately 3 hours. Valacyclovir is excreted with the urine, mainly as acyclovir (more than 80% of the dose) and its metabolite 9-carboxymethoxymethylguanine, less than 1% of the drug is excreted unchanged.
Pharmacokinetics in special clinical cases
In patients with terminal renal failure the T1/2 of acyclovir is approximately 14 hours.
The pharmacokinetics of acyclovir is not significantly impaired in patients infected with Herpes simplex and Varicella zoster viruses, as well as in elderly patients and patients with cirrhosis.
In patients with severe renal impairment, the Cmax of acyclovir is approximately twice that of healthy patients and the T1/2 of acyclovir is 5 times longer.Acyclovir, the main metabolite of valacyclovir, is excreted with breast milk.
After oral administration of valacyclovir at a dose of 500 mg, the Cmax of acyclovir in breast milk was 0.5-2.3 times (1.4 times on average) higher than its corresponding concentrations in maternal plasma. The area under the concentration-time curve (AUC) of acyclovir in breast milk versus the AUC of acyclovir in maternal plasma was 1.4-2.6 (mean 2.2).
The mean concentration of acyclovir in breast milk was 2.24 µg/mL. If the mother is given Valacyclovir at a dose of 500 mg twice daily, the baby will have the same exposure to acyclovir as if it were given orally at a dose of about 0.61 mg/kg/day. The T1/2 of acyclovir from breast milk is the same as that from blood plasma. Valacyclovir in unchanged form is not detected in the mother’s plasma, breast milk or urine.
In late pregnancy, the sustained daily AUC after taking 1 g of valacyclovir was approximately 2 times greater than that after taking acyclovir at a dose of 1.2 g per day. The pharmacokinetic characteristics of valacyclovir do not change in pregnancy.
The administration of valacyclovir in doses of 1 g and 2 g does not impair the distribution and pharmacokinetic characteristics of valacyclovir in HIV-infected patients compared to healthy individuals.
In recipients of organ transplants receiving valacyclovir at a dose of 2 g 4 times daily, the Cmax of acyclovir is equal to or greater than that of healthy volunteers receiving the same dose of the drug, and their daily AUCs are significantly higher.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
1 film-coated tablet contains:
the active substance:
valacyclovir hydrochloride hydrate 611.70 mg, corresponding to 500 mg of valacyclovir;
excipients:
Microcrystalline cellulose 59.60 mg,
povidone-K30 24.50 mg,
magnesium stearate 4.20 mg;
film coating:
Opadray white Y-5-7068 (hypromellose ZsP 7.35 mg, hyprolose 6.3 mg, titanium dioxide 4.2 mg, macrogol/PEG 400 2.1 mg, hypromellose 50sP 1.05 mg) – 21 mg.
How to take, the dosage
How to take, the dosage
Valvir is taken orally. Adults.
Herpes zoster – 1000 mg 3 times a day for 7 days.
Simple herpes – 500 mg 2 times a day. In case of relapses, the course should be 3 or 5 days. In case of the first episode with a severe course the duration of treatment may be increased up to 10 days (in relapses it is ideal to prescribe Valvir in the prodromal period or when the first symptoms of the disease appear, i.e. tingling, itching, burning).
For therapy of labial herpes, it is effective to prescribe the drug in a dose of 2 g 2 times for 1 day: the second dose should be taken approximately 12 hours (but not earlier than 6 hours) after the first dose (do not use this dosing regimen over 1 day, because it has been shown to have no additional clinical benefit).
Prophylaxis of recurrent infections caused by herpes simplex virus: in patients with preserved immunity, 500 mg once daily; with very frequent recurrences (10 or more per year), 250 mg twice daily; for adult patients with immunodeficiency, 500 mg twice daily. Course duration is 4-12 months.
Prevention of genital herpes infection of a healthy partner: infected heterosexual adults with preserved immunity and with the number of exacerbations up to 9 per year – 500 mg once daily for 1 year or more, every day with regular sexual intercourse, with irregular sexual intercourse Valvir should be started 3 days before intended sexual contact (data on prevention of infection in other patient populations are not available).
Prophylaxis of cytomegalovirus infection: to adults and adolescents over 12 years old – 2 g 4 times a day (as soon as possible, after transplantation). Course duration is 90 days, but in high-risk patients treatment may be longer. It is recommended to reduce the dose of Valvir in patients with significant reduction of renal function.
Interaction
Interaction
No clinically significant interactions were found.
Cimetidine and probenecid after taking 1 g of valacyclovir increase AUC of acyclovir, reducing its renal clearance (but there is no need to adjust the dose of valacyclovir because of the wide therapeutic index of acyclovir).
Caution should be exercised in case of concomitant use of valacyclovir in high doses (4 g/day) and drugs that compete with acyclovir for the elimination route (the latter is eliminated unchanged in the urine due to active tubular secretion), because there is a potential risk of increased plasma levels of one or both drugs or their metabolites.
In concomitant use of acyclovir with mycophenolate mofetil, an increase in AUC of the former and inactive metabolite of the latter was noted.
We should also use caution when combining valacyclovir in high doses (4 g/day or more) with drugs that affect renal function (e.g., cyclosporine, tacrolimus).
Special Instructions
Special Instructions
Administration of the drug in high doses for a long time in conditions accompanied by severe immunodeficiency (bone marrow transplantation, clinically expressed forms of HIV infection, kidney transplantation) resulted in the development of thrombocytopenic purpura and hemolytic-uremic syndrome, up to fatal outcome. In case of side effects on the central nervous system (including agitation, hallucinations, confusion, delirium, convulsions and encephalopathy) the drug should be discontinued.
Patients at risk of dehydration, especially elderly patients, should be adequately hydrated during treatment with Valvir. Patients with renal insufficiency have an increased risk of neurological complications.
In patients with mild to moderate hepatic cirrhosis (synthetic liver function is preserved), no dose adjustment of Valvir is required. No data have been obtained in pharmacokinetic studies in patients with severe cirrhosis (with impaired hepatic synthetic function and the presence of shunts between the portal system and the common vascular system) that suggest the need for dosage adjustment; however, clinical experience with Valvir administration in this category of patients is limited. There are no data on the use of Valvir at high doses (4 g/day or more) in patients with liver disease; therefore, caution should be exercised when prescribing the drug at high doses for this category of patients.
Patients in the elderly do not require dose adjustment except in cases of significant renal impairment. Adequate water-electrolyte balance should be maintained.
Special studies to study the effect of Valvir in patients with liver transplantation have not been conducted. However, prophylactic administration of acyclovir in high doses has been shown to reduce cytomegalovirus infection. Suppressive therapy with Valvir reduces the risk of genital herpes transmission, but does not eliminate it completely and does not lead to a complete cure. During therapy with Valvir, the patient must take measures to ensure the safety of the partner during sexual intercourse.
Pediatric use
There is no clinical experience with the drug in children.
Influence on ability to drive and/or other machinery
Caution should be exercised in case of adverse reactions affecting speed of psychomotor reactions.
Contraindications
Contraindications
With caution: hepatic failure (high doses of the drug), renal failure, pregnancy, lactation.
Side effects
Side effects
The most common adverse reactions when using valacyclovir are: headache and nausea, more serious adverse reactions: thrombotic thrombocytopenic purpura/hemolytic uremic syndrome, acute renal failure and neurological disorders.
The undesired reactions are listed below in accordance with the classification by major systems and organs and by frequency of occurrence: very common – â¥1/10; common – â¥1/100 or Blood and lymphatic system: very rare – leukopenia (mainly in patients with reduced immunity), thrombocytopenia.
Immune system disorders: very rare – anaphylaxis.
Mental disorders and disorders of the nervous system: frequently – headache; sometimes – agitation, including aggressive behavior; rarely – dizziness, confusion, hallucinations, decreased mental capacity; very rarely – agitation, tremor, ataxia, dysarthria; psychotic symptoms, including mania; depression, seizures, encephalopathy, coma.
The symptoms listed are reversible and are usually seen in patients with impaired renal function or with a background of other diseases. Transplant patients receiving high doses of valacyclovir (8 g/day) to prevent CMV infection develop neurologic reactions more often than those receiving lower doses.
Respiratory and mediastinal side: sometimes – dyspnea.
Skin and subcutaneous tissue: sometimes – rashes, including photosensitivity; rarely – itching.
Allergic reactions: very rare – urticaria, angioedema.
Urinary system disorders: rare – renal impairment; very rarely – acute renal failure, renal colic (may be associated with renal impairment).
Others: in patients with severe immune disorders, especially in patients with a far advanced stage of acquired immune deficiency syndrome receiving high doses of valacyclovir (8 g/day) for a long time, there were cases of renal failure, microangiopathic hemolytic anemia and thrombocytopenia (sometimes in combination). Similar complications have been noted in patients with the same disease but not receiving valacyclovir.
The incidence of some adverse reactions cannot be determined from the available data.
Senses: visual impairment.
Blood organs: neutropenia, aplastic anemia, leukoplastic vasculitis, thrombotic thrombocytopenic purpura.
Skin side: erythema multiforme.
Laboratory measures: decrease of hemoglobin, hypercreatininemia.
Other: dysmenorrhea, arthralgia, nasopharyngitis, respiratory tract infections, facial edema, increased blood pressure, tachycardia, fatigue; additionally in children, fever, dehydration, rhinorrhea.
Overdose
Overdose
There are currently insufficient data on valacyclovir overdose.
Symptoms: A single overdose of acyclovir up to 20 g, which was partially absorbed from the gastrointestinal tract, was not accompanied by toxic effects of the drug. Nausea, vomiting, headache, confusion occurred if ultra-high doses of acyclovir were taken orally for several days; when administered intravenously – increased serum creatinine concentration, development of renal failure, confusion, hallucinations, agitation, seizures, coma.
Treatment: patients should be under close medical supervision for signs of toxic effects. Hemodialysis significantly enhances the removal of acyclovir from the blood and may be considered the method of choice in the management of patients with valacyclovir overdose.
Pregnancy use
Pregnancy use
Pregnant use is possible if the expected effect of therapy for the mother exceeds the potential risk to the fetus (there is not enough information about the use in pregnancy).
Acyclovir, the main metabolite of valacyclovir, is excreted with the breast milk.
Breastfeeding during therapy with valacyclovir is possible if the expected effect of therapy for the mother exceeds the potential risk to the baby.
Similarities
Similarities
Additional information
| Weight | 0.043 kg |
|---|---|
| Shelf life | 2 years |
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Balkanfarma – Dupnitsa AD, Bulgaria |
| Medication form | pills |
| Brand | Balkanfarma – Dupnitsa AD |
Other forms…
Related products
Buy Valvir, 500 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.