No products in the cart.
Vagiferon, vaginal suppositories 10 pcs
€12.80 €11.20
Description
Pharmacodynamics
Combination drug for intravaginal use.
Vagiferon has antiviral, immunomodulatory, anti-inflammatory, antifungal, antimicrobial and antiprotozoal (trichomonacid) action.
Interferon alfa-2b has pronounced antiviral, immunomodulatory properties.
Metronidazole is an antiprotozoal and antibacterial drug, a derivative of 5-nitroimidazole. The mechanism of action consists in biochemical reduction of the 5-nitrogroup of metronidazole by intracellular transport proteins of anaerobic microorganisms and protozoa. The reduced 5-nitrogroup of metronidazole interacts with the DNA of microorganisms, inhibiting the synthesis of nucleic acids, which leads to the death of the bacteria. It is active against Trichomonas vaginalis, Gardnerella vaginalis, as well as Gram-negative anaerobes Bacteroides spp. (including B. fragilis, B. distasonis, B. status, B. thetaiotaomicron, B. vulgaris), Fusobacterium spp. and some Gram-positive anaerobes (sensitive strains of Eubacterium spp, Clostridium spp., Peptococcus niger., Peptostreptococcus spp., Mobiluncus spp.). Aerobic microorganisms and facultative anaerobes are insensitive to metronidazole.
Fluconazole has a highly specific fungicidal (antifungal) action. It is active against mycoses, including those caused by Candida spp. (including generalized forms of candidiasis against immunosuppression), Cryptococcus neoformans and Coccidioides immitis, Microsporum spp., Blastomyces dermatitidis. When used intravaginally it is particularly active against Candida albicans, to a lesser extent against Candida glabrata.
Pharmacokinetics
After intravaginal administration the bioavailability of metronidazole is 20% compared to oral administration. After intravaginal administration it undergoes systemic absorption (about 56%). It is metabolized in the liver by hydroxylation, oxidation and glucuronidation. The activity of the main metabolite (2-oxymetronidazole) is 30% of the activity of the parent compound. T1/2 is 6-12 hours. It is excreted 40-70% (about 20% – in unchanged form) through the kidneys. Pharmacokinetics of interferon and fluconazole with intravaginal route of administration has not been studied
.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
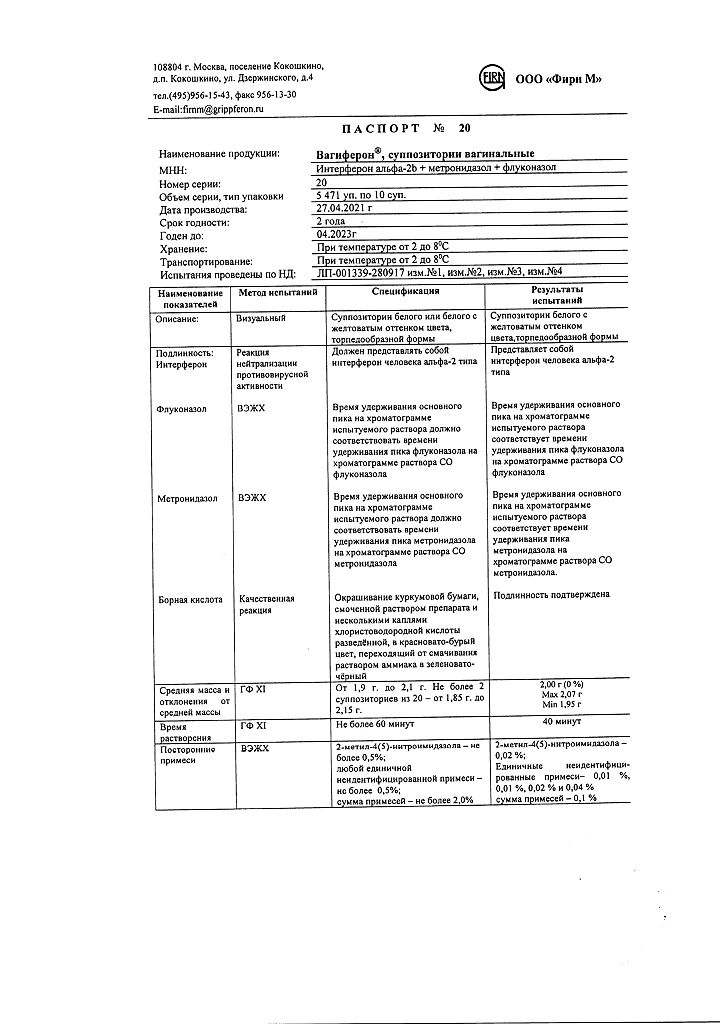
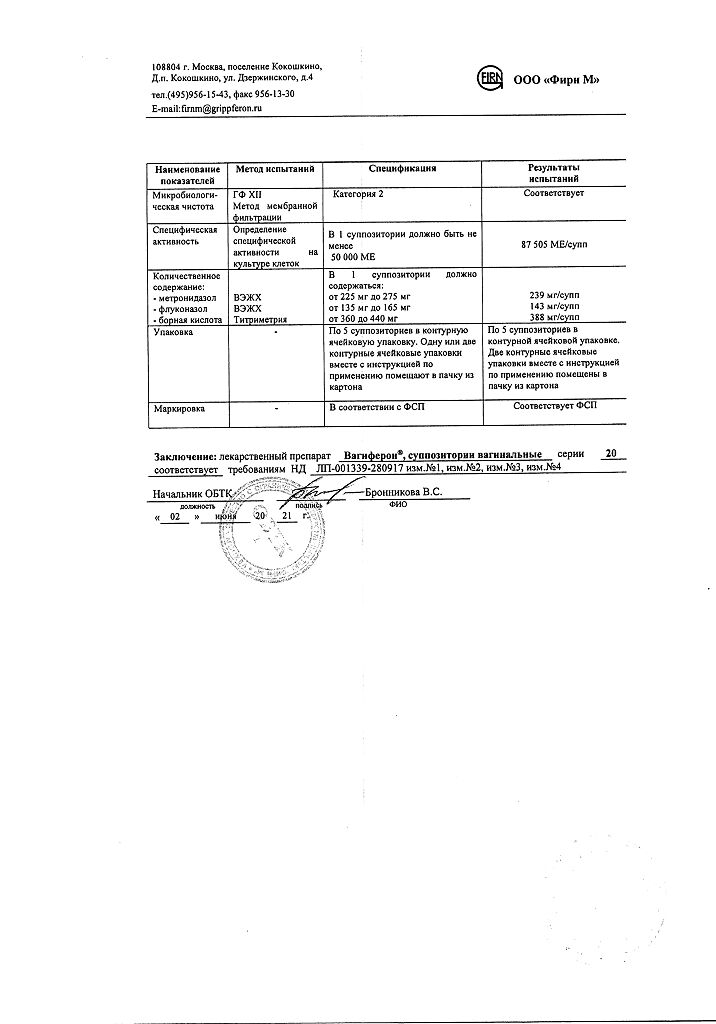
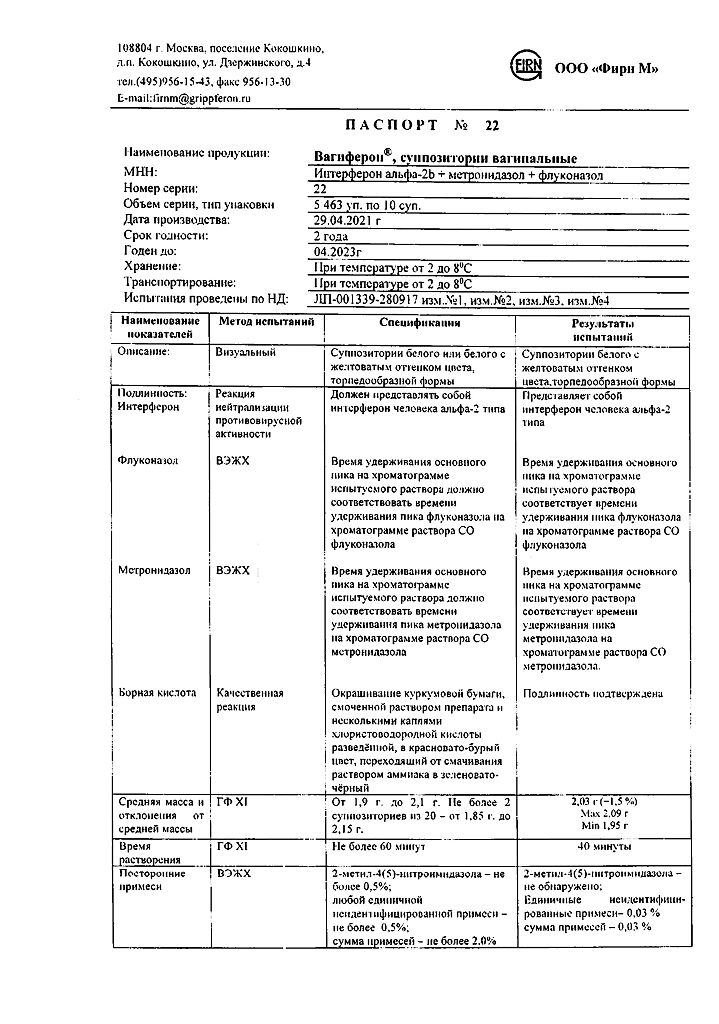
1 vaginal suppository contains:
acting substances:
interferon alpha-2b human recombinant at least 50,000 IU,
metronidazole 250 mg,
Fluconazole 150 mg,
excipients:
boric acid, 400 mg;
dinatrium edetate, 2 mg;
base (macrogol 1500 – 92%, macrogol 400 – 8%) – up to 2 g
How to take, the dosage
How to take, the dosage
Intravaginally. 1 suppository in the evening (before going to bed), for 10 days.
Interaction
Interaction
It is not recommended to combine with nondepolarizing myorelaxants (vecuronium bromide).
Combination with the antibiotic macrolide (Josamycin) of systemic action is possible.
Special Instructions
Special Instructions
At the time of treatment with Vagiferon it is recommended to refrain from sexual intercourse.
If the drug is used together with metronidazole for oral administration, especially during repeated course, it is necessary to monitor the peripheral blood picture (danger of leukopenia).
Ethanol administration is contraindicated during treatment (development of disulfiram-like reactions is possible: spastic abdominal pain, nausea, vomiting, headache, sudden “rush” of blood to the face).
Metronidazole should not be given to patients who have taken disulfiram in the last 2 weeks.
Impact on driving and operating machinery
The drug Vagiferon does not affect the ability to drive vehicles and engage in other potentially dangerous activities requiring increased concentration and rapid psychomotor reactions.
Contraindications
Contraindications
Side effects
Side effects
No adverse effects have been identified with topical use.
In isolated cases allergic reactions and local reactions are possible.
The side effects typical for metronidazole because its systemic absorption is 56%: allergic reactions (rare), leukopenia, ataxia, mental changes (anxiety, mood lability), seizures, rarely: diarrhea, constipation, dizziness, headache, loss of appetite, nausea, vomiting, abdominal pain and cramps, change in taste (rare), dry mouth, “metallic” or unpleasant taste, increased fatigue.
Overdose
Overdose
Cases of overdose of the drug have not been identified.
In case of metronidazole overdose nausea, vomiting, abdominal pain, diarrhea, itching, metallic taste in the mouth, ataxia, dizziness, paresthesias, seizures, leukopenia, dark staining of urine are possible.
Pregnancy use
Pregnancy use
The drug is not used in pregnancy.
Metronidazole penetrates into breast milk.
Breast-feeding while taking the drug should be canceled.
Breast-feeding should be resumed at least 48 hours after the end of the drug.
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At 2-8 °C |
| Manufacturer | Firn M, Russia |
| Medication form | vaginal suppositories |
| Brand | Firn M |
Related products
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Gynecology and Obstetrics
Buy Vagiferon, vaginal suppositories 10 pcs with delivery to USA, UK, Europe and over 120 other countries.