No products in the cart.
Description
Pharmacotherapeutic group
Antibacterials other.
The ATX code: J01ХХ01
Pharmacological properties
Pharmacodynamics
Uronormin-F contains phosphomycin [mono (2-ammonium-2-hydroxymethyl-1,3-propanediol)(2R-cis)-(3-methyloxyranil) phosphonate], a broad spectrum antibacterial agent, a phosphonic acid derivative intended to treat urinary tract infections.
The mechanism of action is associated with inhibition of the first stage of synthesis of the bacterial cell wall. Being a structural analogue of phosphoenolpyruvate it competitively inhibits irreversibly the enzyme UDF-N-acetylglucosamienolpyruviltransferase which catalyzes the reaction of formation of UDF-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine from phosphoenolpyruvate and UDF-N-acetyl-D-glucosamine. The drug is also able to reduce bacterial adhesion to the bladder mucosa, which can play the role of a predisposing factor for recurrent infections. The mechanism of action of the drug explains the absence of cross-resistance with other classes of antibiotics and mutual reinforcement of action with antibiotics of other classes, such as beta-lactam antibiotics.
Phosphomycin is active against a wide range of Gram-positive and Gram-negative microorganisms commonly isolated in urinary tract infections such as Escherichia coli, Citrobacter spp, Klebsiella spp, Proteus spp, Serratia spp, Pseudomonas aeruginosa, Enterrococcus faecalis.
The emergence of resistance in the laboratory is explained by mutations in the glpT and uhp genes, which control L-alpha-glycerophosphate and glucose phosphate transport, respectively.
Pharmacokinetics
Intake:
In oral administration phosphomycin is well absorbed from the intestine and achieves bioavailability of about 50%. Maximum concentration in plasma is observed 2-2.5 hours after oral administration and is 22-32 mg/l. The plasma elimination half-life is 4 hours. Intake with food slows absorption without affecting the concentration in the urine.
Distribution:
Phosphomycin is distributed in the kidneys, bladder wall, prostate and seminal glands. A steady urinary concentration of fosfomycin exceeding the Minimum Bacteriostatic Concentration (MBc) is reached 24-48 hours after oral administration. Fosfomycin does not bind to plasma proteins and crosses the placental barrier. After a single administration, fosfomycin is excreted into breast milk in small amounts.
Elimation:
Phosphomycin is excreted unchanged, mainly by the kidneys, through glomerular filtration (40-50 % of the dose taken is found in the urine), with a half-life of about 4 hours, and, to a lesser extent, with the feces (18-28 % of the dose). The occurrence of a second peak serum concentration 6 and 10 hours after drug administration suggests that the drug is subject to intestinal hepatic recirculation.
The pharmacokinetic properties of fosfomycin are independent of age and pregnancy. The drug cumulates in patients with renal insufficiency; a linear relationship between pharmacokinetic parameters of fosfomycin and glomerular filtration rate has been established.
Indications
Indications
Acute uncomplicated infections of the lower urinary tract (acute cystitis) in women aged 18 years and older, caused by pathogenic microorganisms sensitive to fosfomycin: Escherichia coli, Enterococcus faecalis. Fosfomycin is not indicated for the treatment of pyelonephritis or perinephric abscess.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Other antibacterial drugs.
ATX code: J01ХХ01
Pharmacological properties
Pharmacodynamics
Uronormin-F contains fosfomycin [mono(2-ammonium-2-hydroxymethyl-1,3-propanediol)(2R-cis)-(3-methyloxyranyl) phosphonate], a broad-spectrum antibacterial agent, a phosphonic acid derivative, intended for the treatment of urinary tract infections.
The mechanism of action is associated with the suppression of the first stage of bacterial cell wall synthesis. Being a structural analogue of phosphoenolpyruvate, it competitively and irreversibly inhibits the enzyme UDP-N-acetylglucosamienolpyruvyltransferase, which catalyzes the formation of UDP-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine from phosphoenolpyruvate and UDP-N-acetyl-D-glucosamine. The drug is also able to reduce the adhesion of bacteria to the mucous membranes of the bladder, which can play the role of a predisposing factor for recurrent infections. The mechanism of action of the drug explains the lack of cross-resistance with other classes of antibiotics and the mutual enhancement of action with antibiotics of other classes, for example with beta-lactam antibiotics.
Fosfomycin is active against a wide range of gram-positive and gram-negative microorganisms usually isolated during urinary tract infections, such as Escherichia coli, Citrobacter spp., Klebsiella spp., Proteus spp., Serratia spp., Pseudomonas aeruginosa, Enterrococcus faecalis.
The emergence of resistance in laboratory conditions is explained by mutations in the glpT and uhp genes, which control the transport of L-alpha-glycerophosphates and glucose phosphates, respectively.
Pharmacokinetics
Suction:
When taken orally, fosfomycin is well absorbed from the intestine and reaches a bioavailability of about 50%. The maximum plasma concentration is observed 2-2.5 hours after oral administration and is 22-32 mg/l. The plasma half-life is 4 hours. Taking with food slows down absorption without affecting urinary concentrations.
Distribution:
Fosfomycin is distributed in the kidneys, bladder walls, prostate and seminal glands. A constant concentration of fosfomycin in urine exceeding the Minimum Bacteriostatic Concentration (MBC) is achieved 24-48 hours after oral administration. Fosfomycin is not bound to plasma proteins and crosses the placental barrier. After a single dose, fosfomycin is excreted into breast milk in small quantities.
Removal:
Fosfomycin is excreted unchanged, mainly by the kidneys, by glomerular filtration (40-50% of the dose taken is found in the urine), with an elimination half-life of about 4 hours, and to a lesser extent in feces (18-28% of the dose). The occurrence of a second peak serum concentration 6 and 10 hours after dosing suggests that the drug is subject to enterohepatic recirculation.
The pharmacokinetic properties of fosfomycin do not depend on age and pregnancy. The drug accumulates in patients with renal failure; A linear relationship has been established between the pharmacokinetic parameters of fosfomycin and glomerular filtration rate.
Special instructions
Special instructions
Precautions
General
For the treatment of acute cystitis, only a single dose of fosfomycin should be used. Repeated daily doses of fosfomycin did not improve clinical success or microbiological eradication rates compared with single-dose therapy, but increased the incidence of adverse reactions.
Hypersensitivity reactions, including anaphylaxis and anaphylactic shock, have been reported during fosfomycin therapy, incl. and life-threatening. If such reactions occur, adequate medical treatment is necessary, and repeated administration of fosfomycin is not recommended.
Before prescribing fosfomycin to patients with severe renal impairment (Cl creatinine < 30 ml/min) or patients on hemodialysis, the benefits of its use should be assessed against the potential risks, since the main route of elimination of fosfomycin is the kidneys.
Diseases associated with Clostridium difficile
Clostridium difficile-associated illnesses have been reported with the use of many antibacterial agents, including fosfomycin. The severity of these diseases can range from mild diarrhea to life-threatening colitis. This diagnosis should be considered when monitoring patients with diarrhea or symptoms of colitis, pseudomembranous colitis, toxic megacolon, or colonic perforation after use of any antibacterial agent. Clostridium difficile-associated illnesses have been reported to occur within 2 months of starting antibacterial therapy.
Treatment with antibacterial drugs can alter the normal flora of the colon and in many cases lead to overgrowth of Clostridium difficile, which produces toxins A and B, which contribute to the development of diseases leading to significant morbidity and mortality. Diseases associated with Clostridium difficile may be refractory to antimicrobial therapy.
If a diagnosis of a disease associated with Clostridium difficile is suspected or confirmed, appropriate therapeutic measures must be initiated. Mild cases of the disease usually respond to discontinuation of antibacterial drugs not directed against Clostridium difficile. In moderate to severe cases, consider administration of saline and electrolytes, protein supplementation, and therapy with an antibacterial agent clinically effective against Clostridium difficile. Drugs that suppress intestinal motility should not be used in the treatment of diseases associated with Clostridium difficile, as they can slow down the elimination of Clostridium difficile and its toxins. Surgical evaluation should be performed as clinically indicated, as some severe cases may require surgery.
Sensitivity/resistance
The use of fosfomycin in the absence of a proven or suspected bacterial infection may be ineffective and lead to the development of drug-resistant bacteria.
Use in pediatrics
The safety and effectiveness of fosfomycin in children under 18 years of age have not been established.
Impact on the ability to drive vehicles and operate machinery. Although no studies have been conducted on the ability to drive or operate machines, dizziness has been reported in patients taking fosfomycin. This may affect some patients’ ability to drive and operate machines.
Active ingredient
Active ingredient
Fosfomycin
Composition
Composition
1 package contains:
Active ingredient:
Fosfomycin trometamol – 5.631 g in terms of fosfomycin – 3.0 g
Excipients:
Sucrose – 2.213 g
Tangerine flavor – 0.070 g
Orange flavor – 0.070 g
Sodium saccharinate – 0.016 g.
Pregnancy
Pregnancy
Fosfomycin crosses the placental barrier and its safety in the treatment of infections during pregnancy has not been established. Fosfomycin should be used during pregnancy only for absolute indications and only after taking into account the balance of potential benefit to the mother and risk to the fetus.
Fosfomycin may be excreted in breast milk; a decision should be made to discontinue breastfeeding or to avoid using fosfomycin, taking into account the potential benefit to the mother and the risk to the newborn.
Contraindications
Contraindications
Hypersensitivity to fosfomycin.
Side Effects
Side Effects
In three studies conducted in the United States, 1233 patients received fosfomycin therapy. The most common adverse reactions occurring in >1% of patients, regardless of association with drug use, were diarrhea (10.4%), headache (10.3%), vaginitis (7.6%), nausea (5.2%), rhinitis (4.5%), back pain (3.0%), dysmenorrhea (2.6%), pharyngitis (2.5%), dizziness (2.3%), abdominal pain (2.2%), pain (2.2%), dyspepsia (1.8%), asthenia (1.7%) and rash (1.4%).
In addition, the following adverse reactions were observed with a frequency of less than 1%: stool disorders, anorexia, constipation, dry mouth, dysuria, hearing loss, fever, flatulence, influenza-like syndrome, hematuria, infections, insomnia, lymphadenopathy, menstrual irregularities, migraine, myalgia, nervousness, paresthesia, itching, skin diseases (disorders). skin) and vomiting.
In the US study population, statistically significant laboratory changes reported without regard to drug association included increased eosinophils, increased or decreased white blood cell counts, increased bilirubin, ALT and AST, ALP, decreased hematocrit, decreased Hb, and increased and decreased platelet counts. Changes were usually transient, clinically insignificant and occurred in less than 1% of patients.
In the same population, studies were conducted of adverse reactions that were considered to be associated with fosfomycin and were observed in more than 1% of patients receiving fosfomycin. These included diarrhea (9%), vaginitis (5.5%), nausea (4.1%), headache (3.9%), dizziness (1.3%), asthenia (1.1%) and dyspepsia (1.1%). The most commonly observed reaction, diarrhea, was considered mild and transient (resolved without any intervention).
One case of unilateral optic neuritis has been reported and was considered likely to be associated with fosfomycin use.
During post-marketing experience with fosfomycin, reports of vulvovaginitis, tachycardia, hearing loss, urticaria and anaphylactic reactions, including anaphylactic shock and hypersensitivity, have been reported. Cases of angioedema, aplastic anemia, asthma (exacerbation), cholestatic jaundice, general loss of taste, hepatic necrosis, metallic taste in the mouth and vestibular disorders have also been reported. One case of toxic megacolon has been reported and was found to be unrelated to fosfomycin.
Interaction
Interaction
Metoclopramide. With the simultaneous use of fosfomycin and metoclopramide, which increases gastrointestinal motility, the concentration of fosfomycin in the blood serum and its excretion in the urine decreases. Other drugs that increase gastrointestinal motility may have a similar effect.
Probenecid. Probenecid should not be co-administered with fosfomycin as it has been shown to significantly reduce the renal clearance and elimination of fosfomycin.
Probenecid, when administered to healthy volunteers receiving an infusion of fosfomycin disodium, significantly reduced renal clearance, probably due to inhibition of tubular secretion, resulting in decreased drug concentrations in urine.
The effect of metoclopramide and probenecid on urinary fosfomycin levels following co-administration with fosfomycin in women with acute urinary tract infection has not been evaluated therapeutically. Based on data obtained from healthy volunteers, urinary concentrations of fosfomycin cannot exceed the bactericidal concentration for a sufficiently long period of time to result in microbiological cure. None of these drugs should be co-administered with fosfomycin.
Cimetidine. Cimetidine does not affect the pharmacokinetics or urinary concentrations of fosfomycin when used together.
Eating. Eating delays the absorption of fosfomycin, which leads to a decrease in Cmax in blood plasma and concentration in urine. Therefore, it is recommended to take fosfomycin tromethamine on an empty stomach or at least 2–3 hours after a meal.
Overdose
Overdose
Data on oral overdose of fosfomycin are limited.
Symptoms: in cases of overdose with parenteral use, hypotension, drowsiness, fluid and electrolyte imbalances, thrombocytopenia and hypoprothrombinemia have been reported.
Treatment: in case of overdose, symptomatic and supportive therapy should be carried out. Urinary excretion of fosfomycin should be stimulated by adequate fluid intake.
Storage conditions
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
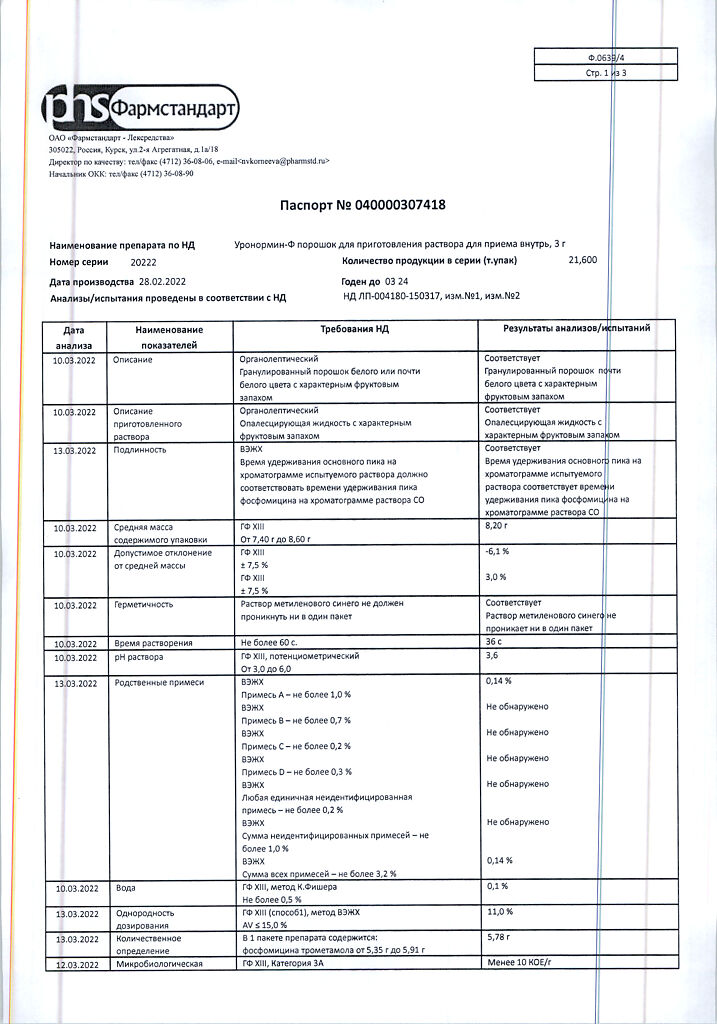
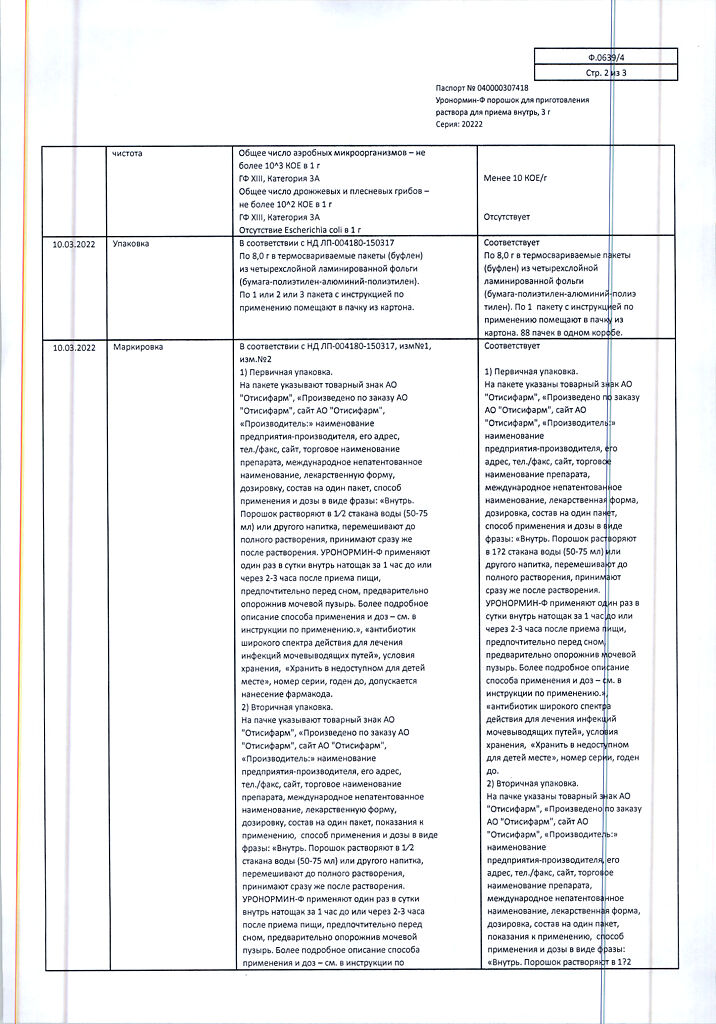
| Shelf life | 2 years. |
|---|---|
| Conditions of storage | Store at temperatures under 25 ° C. Keep out of reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | oral solution |
| Brand | Pharmstandard-Leksredstva |
Related products
Buy Uronormin-F, 3 g 8 g with delivery to USA, UK, Europe and over 120 other countries.