No products in the cart.
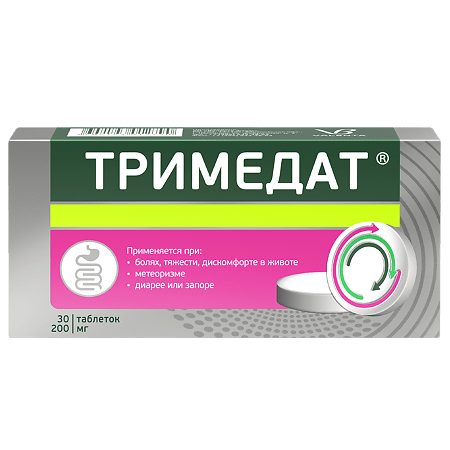
Trimedat, tablets 200 mg 30 pcs
€23.57 €19.57
Description
Spasmolytic agent.
ATX code: A03AA05
Pharmacological properties
Pharmacodynamics
Trimebutine, acting on the enkephalinergic system of the intestine, is a regulator of its peristalsis. Acting on peripheral δ-, μ- and k-receptors including those located directly on the smooth muscles throughout the gastrointestinal tract (GIT), it regulates motility without affecting the central nervous system. Thus, trimebutine restores normal physiological activity of intestinal muscles in various GI diseases associated with motility disorders.
Normalizing visceral sensitivity, trimebutine provides analgesic effect in abdominal pain syndrome.
Pharmacokinetics
Trimebutin is rapidly absorbed from the gastrointestinal tract after oral administration. Maximum concentration (Cmax) in blood plasma is reached after 1-2 hours. Bioavailability is 4-6%. Volume of distribution (Vd) is 88 l. The degree of binding to plasma proteins is low – about 5%. Trimebutine penetrates through the placental barrier to a small extent. Trimebutine is biotransformed in the liver and excreted in the urine mainly as metabolites (about 70% during the first 24 hours). The elimination half-life (T1/2) is about 12 hours.
Indications
Indications
Symptomatic treatment of pain, cramps and discomfort in the abdomen, sensation of bloating (flatulence), intestinal motor disorders with changes in stool frequency (diarrhea or constipation), dyspepsia, heartburn, belching, nausea, vomiting associated with functional diseases of the gastrointestinal tract and biliary tract (non-erosive form of gastroesophageal reflux disease; cholelithiasis; biliary tract dysfunction; irritable bowel syndrome; sphincter of Oddi dysfunction, postcholecystectomy syndrome).
Postoperative paralytic ileus.
Pharmacological effect
Pharmacological effect
Antispasmodic.
ATX code: A03AA05
Pharmacological properties
Pharmacodynamics
Trimebutine, acting on the enkephalinergic system of the intestine, is a regulator of its peristalsis. Acting on peripheral δ-, μ- and k- receptors, including those located directly on smooth muscles throughout the gastrointestinal tract (GIT), it regulates motility without affecting the central nervous system. Thus, trimebutine restores the normal physiological activity of the intestinal muscles in various gastrointestinal diseases associated with motility disorders.
By normalizing visceral sensitivity, trimebutine provides an analgesic effect for abdominal pain syndrome.
Pharmacokinetics
After oral administration, trimebutine is rapidly absorbed from the gastrointestinal tract. The maximum concentration (Cmax) in blood plasma is achieved after 1-2 hours. Bioavailability is 4-6%. Distribution volume (Vd) – 88 l. The degree of binding to plasma proteins is low – about 5%. Trimebutine penetrates the placental barrier to a small extent. Trimebutine is biotransformed in the liver and excreted in the urine mainly in the form of metabolites (approximately 70% during the first 24 hours). The half-life (T1/2) is about 12 hours.
Special instructions
Special instructions
A course of treatment for irritable bowel syndrome in the acute period of 600 mg per day for 4 weeks and continuation of treatment after the course at a dose of 300 mg per day for 12 weeks helps to avoid relapse of the disease.
Active ingredient
Active ingredient
Trimebutine
Composition
Composition
One tablet contains:
active ingredient: trimebutine maleate – 200 mg;
excipients: lactose monohydrate, potato starch, povidone, colloidal silicon dioxide (aerosil), magnesium stearate, talc.
Pregnancy
Pregnancy
Experimental studies have not revealed any evidence of teratogenicity or embryotoxicity of the drug. However, due to the lack of necessary clinical data, the use of Trimedat® during pregnancy is contraindicated.
It is not recommended to prescribe Trimedat® during lactation due to the lack of reliable clinical data confirming the safety of the drug during this period. If it is necessary to use the drug during lactation, breastfeeding should be stopped.
Contraindications
Contraindications
Hypersensitivity to the components included in the drug.
Lactase deficiency, lactose intolerance, glucose-galactose malabsorption.
Children under 3 years of age (for this dosage form).
Pregnancy
Precautions for use
The drug Trimedat® should be used with caution during breastfeeding, since there is no data on its ability to pass into breast milk.
Side Effects
Side Effects
From the digestive system: dry mouth, unpleasant taste, diarrhea, dyspepsia, nausea, constipation.
From the nervous system: drowsiness, fatigue, dizziness, headache, anxiety.
Allergic reactions: skin rash.
Other: menstrual irregularities, painful enlargement of the mammary glands, urinary retention.
Interaction
Interaction
Drug interactions with Trimedat® have not been described.
Overdose
Overdose
Cases of overdose of the drug Trimedat®
not registered to date.
Treatment: discontinuation of the drug, gastric lavage, administration of activated charcoal, symptomatic therapy. There are no specific antidotes.
Storage conditions
Storage conditions
In original packaging at a temperature not exceeding 25 oC.
Keep out of the reach of children.
Shelf life
Shelf life
3 years.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Valenta Pharm JSC, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In the original package at a temperature not exceeding 25 oC. Store out of the reach of children. |
| Manufacturer | Valenta Farm, Russia |
| Medication form | pills |
| Brand | Valenta Farm |
Other forms…
Related products
Buy Trimedat, tablets 200 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.