No products in the cart.
Trental, 100 mg 60 pcs
€20.81 €17.67
Description
Pharmacodynamics
The drug improving microcirculation, angioprotector, xanthine derivative. Trental improves the rheological properties of the blood (fluidity) by influencing the pathologically changed deformability of erythrocytes, inhibiting platelet aggregation and reducing the increased blood viscosity. It improves microcirculation in the areas of disturbed blood circulation.
The mechanism of action of pentoxifylline is associated with inhibition of phosphodiesterase and accumulation of CAMF in vascular smooth muscle cells and blood cells.
With a weak myotropic vasodilatory effect, pentoxifylline slightly decreases RPS and slightly dilates coronary vessels.
The treatment with Trental leads to improvement of the symptomatology of cerebral circulatory disorders.
The success of treatment in peripheral arterial occlusive disease (e.g., intermittent claudication) is seen in prolongation of walking distance, elimination of night cramps in the calf muscles and disappearance of pain at rest.
Pharmacokinetics
Intake
After oral administration pentoxifylline is almost completely absorbed from the GI tract.
Bioavailability averages 19%.
Metabolism
The main pharmacologically active metabolite 1-(5-hydroxyhexyl)-3,7-dimethylxanthine is determined in the plasma at a concentration twice that of the unchanged substance and is in reversible biochemical equilibrium with it. For this reason pentoxifylline and its metabolite should be considered as an active whole, therefore, it can be assumed that the bioavailability of the active substance is significantly higher. Pentoxifylline is completely biotransformed in the body.
The T1/2 of pentoxifylline is 1.6 h.
More than 90% is excreted by the kidneys as unconjugated water-soluble polar metabolites.
Pharmacokinetics in special clinical cases
In patients with severe renal impairment the excretion of metabolites is delayed.
In patients with impaired liver function, prolongation of T1/2 of pentoxifylline and increased bioavailability have been noted.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
Active ingredients:
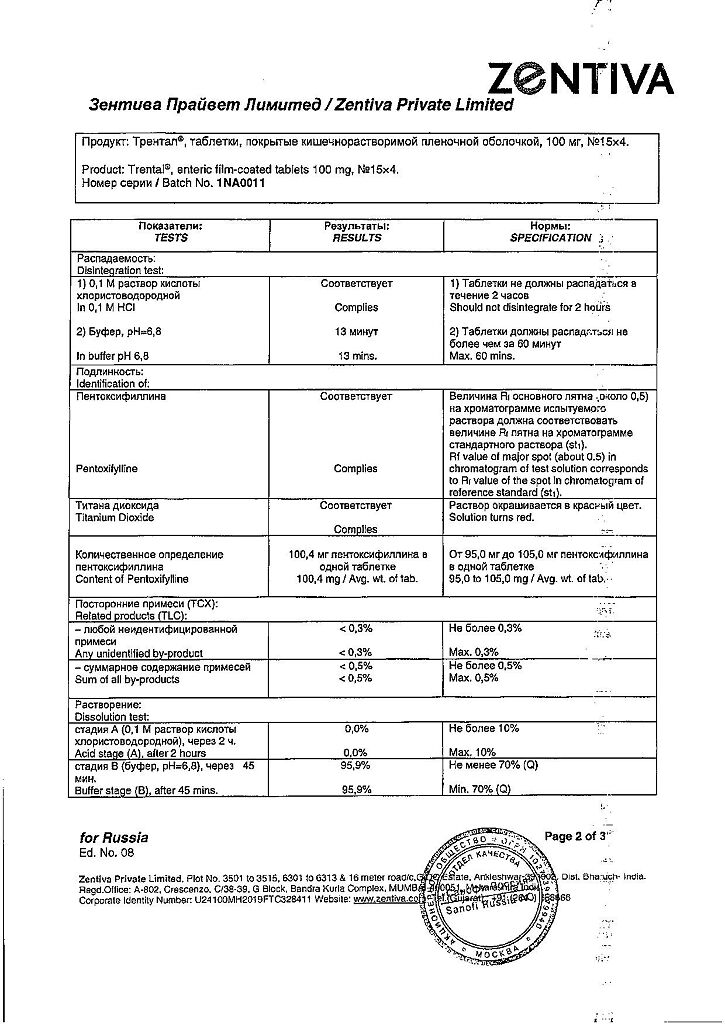
Pentoxifylline 100 mg.
Auxiliary substances:
lactose,
starch,
Talc,
Colloidal silica,
Magnesium stearate.
Composition of the shell:
methacrylic acid copolymer,
sodium hydroxide,
Macrogol (polyethylene glycol) 8000,
Talc,
Titanium dioxide (E171).
How to take, the dosage
How to take, the dosage
The dose is set individually, according to the patient’s characteristics.
The drug is given orally at 100 mg (1 tablet) 3 times a day, with subsequent gradual increase of the dose to 200 mg 2-3 times a day. Maximum single dose is 400 mg. Maximal daily dose is 1200 mg. Tablets should be swallowed in whole during or immediately after a meal, with plenty of water.
In patients with impaired renal function (CKI less than 30 ml/min), the dose should be reduced to 1-2 tablets/day.
In patients with severe hepatic impairment the dose should be reduced taking into account individual tolerance of the drug.
In patients with low BP and also in those at risk due to possible BP decrease (patients with severe CHD or with hemodynamically significant cerebral stenosis) the treatment can be started in small doses; in these cases the dose should be increased gradually.
Interaction
Interaction
Pentoxifylline may increase the effect of drugs that reduce blood pressure (ACE inhibitors, nitrates).
Pentoxifylline may increase the effect of drugs that affect the clotting system (indirect and direct anticoagulants, thrombolytics), antibiotics (including cephalosporins).
Cimetidine increases the plasma concentration of pentoxifylline (risk of side effects).
The co-administration with other xanthines may lead to excessive jitters.
The hypoglycemic effect of insulin or oral hypoglycemic agents may be enhanced when pentoxifylline is taken (increased risk of hypoglycemia). If combined therapy is necessary, close monitoring of patients is required.
In some patients, concomitant administration of pentoxifylline and theophylline may lead to increased plasma concentrations of theophylline. This may increase or intensify theophylline-related side effects.
Special Instructions
Special Instructions
Hypatic disorders
In patients with severe hepatic impairment the T1/2 of pentoxifylline is prolonged. Use with caution in patients with severe hepatic impairment (risk of cumulation and increased risk of side effects).
The use in renal impairment
In severe renal impairment, the excretion of metabolites is delayed. Use the drug with caution in patients with impaired renal function – CKR less than 30 ml/min (risk of cumulation and increased risk of side effects).
Particular instructions
The treatment should be performed under control of BP.
In diabetic patients taking hypoglycemic agents, administration of the drug in high doses may cause marked hypoglycemia (dose adjustment is required).
When prescribing concomitantly with anticoagulants, the parameters of the blood coagulation system should be closely monitored.
In patients who have recently undergone surgery, systematic monitoring of hemoglobin and hematocrit levels is necessary.
In the elderly, a dose reduction may be required (increased bioavailability and decreased excretion rate).
Smoking may decrease the therapeutic effectiveness of the drug.
Pediatric use
The safety and effectiveness of pentoxifylline in children have not been adequately studied.
Contraindications
Contraindications
With caution should be used in patients with: severe cardiac rhythm disturbances (risk of worsening arrhythmia), arterial hypotension (risk of further BP decline), chronic heart failure, gastric and duodenal ulcer disease, renal impairment – CKR less than 30 ml/min (risk of cumulation and increased risk of side effects), severe liver function disturbances (risk of cumulation and increased risk of side effects), increased tendency to bleeding, incl.as a result of anticoagulant use or in case of disorders of the blood coagulation system (risk of more severe bleeding), after recent surgical interventions.
Side effects
Side effects
CNS disorders: headache, dizziness, anxiety, sleep disturbances, seizures; very rare – aseptic meningitis.
Dermatological reactions: facial hyperemia, blood rushes to the skin of the face and upper chest, edema, increased nail fragility.
Digestive system disorders: xerostomia, anorexia, bowel atony, feeling of pressure and overflow in the stomach, nausea, vomiting, diarrhea; in single cases – intrahepatic cholestasis and increased activity of liver transaminases (AST, ALT), ALP (alkaline phosphatase).
Cardiovascular system: tachycardia, arrhythmia, cardialgia, progression of angina pectoris, decreased blood pressure.
Hematopoietic system: leukopenia, thrombocytopenia, pancytopenia, bleeding from blood vessels of the skin, mucous membranes, stomach, intestines, hypofibrinogenemia.
VIight: visual impairment, scotoma.
Allergic reactions: itching, skin hyperemia, urticaria, angioedema, anaphylactic shock.
Side effects are possible when using Trental in high doses.
Overdose
Overdose
Symptoms: nausea, dizziness, tachycardia, BP decrease, arrhythmia, skin hyperemia, chills, loss of consciousness, areflexia, tonic-clonic convulsions.
Treatment: if necessary, symptomatic therapy shall be carried out. The patient should be placed in horizontal position with elevated legs. A specific antidote is unknown. Monitoring of vital body functions and general measures, aimed at their maintenance, monitor airway patency. In case of seizures, diazepam is used.
Similarities
Similarities
Additional information
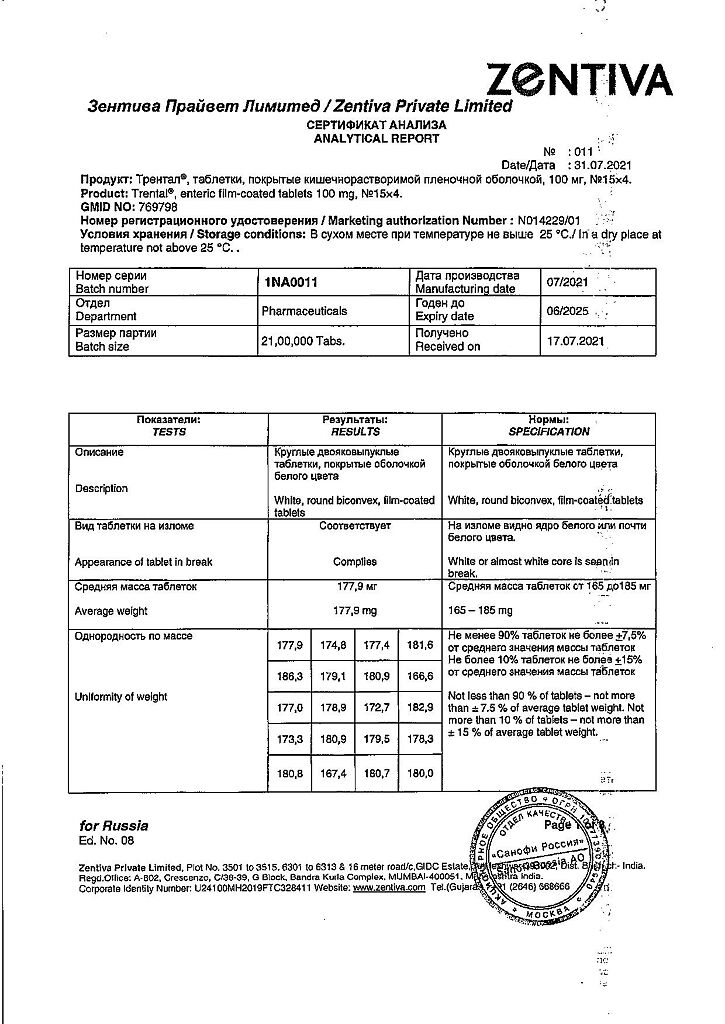
| Shelf life | 4 years. |
|---|---|
| Conditions of storage | Store in a dry place at a temperature not exceeding 25 °C. |
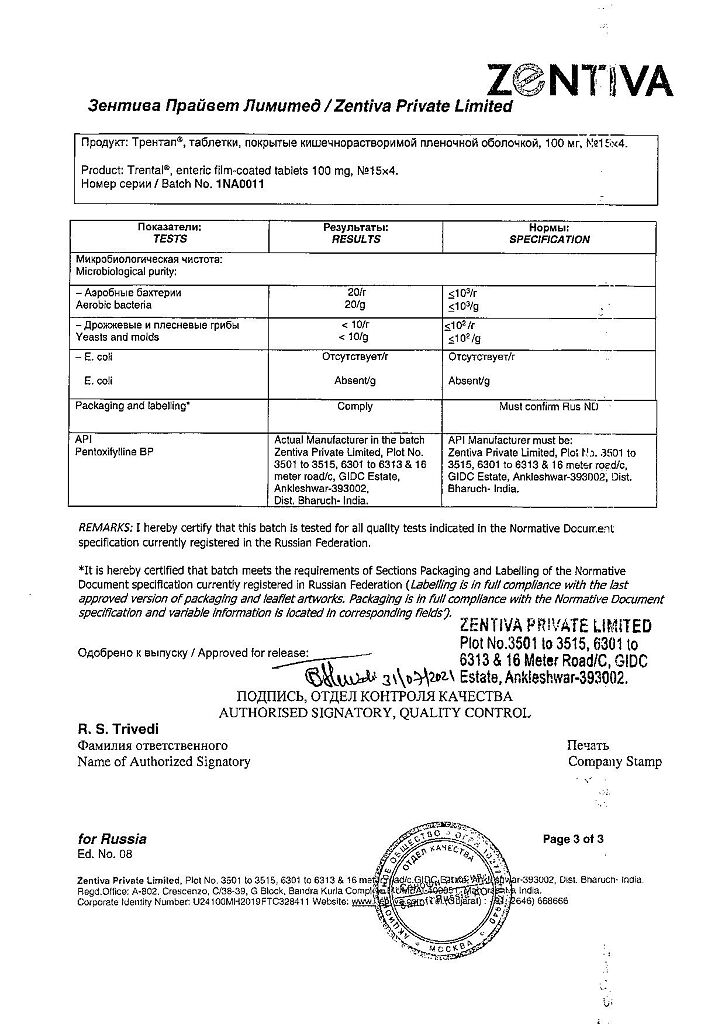
| Manufacturer | Zentiva Private Limited, India |
| Medication form | enteric soluble tablets |
| Brand | Zentiva Private Limited |
Related products
Buy Trental, 100 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.