No products in the cart.
Description
Pharmacotherapeutic group: antitumor drug – alkaloid
ATX code: L01XX17
Pharmacological properties
Pharmacodynamics
Antitumor drug, topoisomerase I inhibitor. Topoisomerase I is an enzyme directly involved in the replication of deoxyribonucleic acid (DNA). Topotecan stabilizes the covalent complex of the enzyme and helical cleaved DNA, which is an intermediate in the catalytic mechanism.
Inhibition of topoisomerase I leads to the breakage of single-helix DNA and stops DNA replication.
Pharmacokinetics
.Distribution
In intravenous (IV) administration of topotecan to adults at doses of 0.5-1.5 mg/m2 as a 30-minute daily infusion for 5 days, the area under the concentration-time curve (AUC) increased in proportion to increasing dose.
The binding of topotecan to plasma proteins is low – 35%. Distribution between blood cells and plasma is homogeneous. Topotecan has a high volume of distribution (Vd) – about 132 l.
When comparing pharmacokinetic parameters, no changes in pharmacokinetics were found during a 5-day course of therapy.
The plasma clearance and Vd values were slightly higher in men than in women. However, these differences were consistent with differences in body surface area.
Metabolism
The main route of metabolism of topotecan is pH-dependent hydrolysis of the lactone ring, in which an open ring carboxylate is formed.
Metabolism is less than 10% of the excreted topotecan. The N-demethylated metabolite of topotecan, which has similar or less activity than topotecan, is detected in the urine, blood plasma and feces. After IV administration, the average AUC of both topotecan and topotecan metabolite was less than 10% for both total topotecan and topotecan in lactone form. In the urine, O-glucuronide of topotecan and N-demethylated topotecan are detected.
In vitro. Topotecan did not inhibit the CYP1A2, CYP2A6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E, CYP3A, CYP4A cytochrome P450 system isoenzymes or the cytosolic enzymes dihydropyrimidinoxidase or xanthine oxidase.
Elimation
After intravenous administration the curve of reduction of plasma concentrations of Topotecan has a biexponential character. The pharmacokinetics of topotecan during IV administration is approximately proportional to the administered dose.
When topotecan is administered by injection into adults at doses of 0.5-1.5 mg/m2 as a 30-minute daily infusion for 5 days, the plasma clearance of topotecan is high at 62 L/h, which is approximately 2/3 of the hepatic blood flow. The half-life (T1/2) is relatively short and is 2-3 h.
After a 5-day course of topotecan, the total excretion of topotecan and its metabolites was 71% to 76% of the IV dose administered. Approximately 51% was excreted through the kidneys as topotecan, 3% – as N-demethylated metabolite of topotecan, 18% of topotecan and 1.7% of the N-demethylated metabolite of topotecan were excreted through the intestines. Overall, as a metabolite (N-demethylated metabolite of topotecan) less than 7% of topotecan is excreted through the kidneys and intestines (range 4% to 9%). Urinary concentrations of O-glucuronide topotecan and N-demethylated O-glucuronide topotecan are less than 2% of the administered dose.
When topotecan is administered in combination with cisplatin (cisplatin on day 1, topotecan on days 1-5), topotecan clearance decreases on day 5 compared with day 1 (19.1 L/h/m2 versus 21.3 L/h/m2, respectively).
In population-based studies, coadministration of granisetron, ondansetron, morphine, or glucocorticosteroids had no significant effect on the pharmacokinetics of topotecan.
Topotecan accumulates in the body in minimal or no amounts with repeated daily intravenous administration, and there are no data on changes in pharmacokinetics with repeated administration.
Pharmacokinetics in Special Clinical Cases
In a population-based study of IV administration of topotecan, a variety of factors, including age, body weight and presence of ascites, had no significant effect on clearance.
Children
The pharmacokinetics of topotecan as a 30-minute infusion over 5 days were evaluated in two studies. One study included a dose range of 1.4 mg/m2 to 2.4 mg/m2 involving children aged 2 to 12 years (n=18), adolescents 12 to 16 years (n=9) and young adults 16 to 21 years (n=9) with solid tumors difficult to treat. The second study included a dose range of 2.0 mg/m2 to 5.2 mg/m2 involving children (n=8), adolescents (n=3) and young adults (n=3) with leukemia.
In both studies, there was no apparent difference in the pharmacokinetic parameters of topotecan among different age groups and also according to disease (solid tumor or leukemia). These data are limited enough to present definite conclusions.
Kidney function impairment
In patients with impaired renal function (creatinine clearance (CK) 41-60 mL/min), the plasma clearance of IV injected topotecan was reduced to approximately 67% of that of the control group. Vd decreased slightly and thus T1/2 increased by only 14%. In patients with moderate renal impairment (CKR 20-39 mL/min), the plasma clearance of topotecan is reduced to 34% of the control value. Vd is reduced by approximately 25%, resulting in an increase in mean T1/2 from 1.9 h to 4.9 h.
Disorders of liver function
. In patients with impaired liver function (serum bilirubin 1.5 mg/dL to 10 mg/dL), plasma clearance of lactone-formed topotecan after IV administration is reduced to approximately 67% of that of the control group and total plasma clearance is reduced by 10% compared with the control group. T1/2 of topotecan is increased by approximately 30%, with no significant change in Vd.
Indications
Indications
– small cell lung cancer;
– ovarian cancer;
– recurrent or persistent cervical cancer that is not amenable to surgical treatment and/or radiation therapy (stage IVB), as part of combination therapy with cisplatin.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antitumor agent – alkaloid
ATX code: L01XX17
Pharmacological properties
Pharmacodynamics
Antitumor drug, topoisomerase I inhibitor. Topoisomerase I is an enzyme directly involved in the replication of deoxyribonucleic acid (DNA). Topotecan stabilizes the covalent complex of the enzyme and helical-cleaved DNA, which is an intermediate link in the catalytic mechanism.
Inhibition of topoisomerase I leads to single-stranded DNA break and stops DNA replication.
Pharmacokinetics
Distribution
When topotecan was administered intravenously (IV) to adults at doses of 0.5-1.5 mg/m2 as a 30-minute daily infusion for 5 days, the area under the concentration-time curve (AUC) increased proportionally with increasing dose.
The binding of topotecan to blood plasma proteins is low – 35%. The distribution between blood cells and plasma is uniform. Topotecan is characterized by a high volume of distribution (Vd) – about 132 l.
When comparing pharmacokinetic parameters, no changes in pharmacokinetics were revealed during the 5-day course of therapy.
The values of blood plasma clearance and Vd were slightly higher in men than in women. However, these differences corresponded with differences in body surface area.
Metabolism
The main route of metabolism of topotecan is pH-dependent hydrolysis of the lactone ring, which produces an open-ring carboxylate.
Less than 10% of excreted topotecan is metabolized. An N-demethylated metabolite of topotecan, which has similar or less activity than topotecan, is found in urine, blood plasma and feces. Following IV administration, the mean AUC of topotecan metabolite and topotecan was less than 10% for both total topotecan and topotecan lactone. O-glucuronide of topotecan and N-demethylated topotecan are found in urine.
In vitro, topotecan did not inhibit the isoenzymes CYP1A2, CYP2A6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E, CYP3A, CYP4A of the cytochrome P450 system, as well as the cytosolic enzymes dihydropyrimidine oxidase or xanthine oxidase.
Removal
After intravenous administration, the curve of decrease in the concentration of topotecan in the blood plasma is biexponential. The pharmacokinetics of topotecan when administered intravenously is approximately proportional to the administered dose.
When topotecan is administered intravenously to adults in doses of 0.5-1.5 mg/m2 as a 30-minute daily infusion for 5 days, the plasma clearance of topotecan is high – 62 l/h, which is approximately 2/3 of the hepatic blood flow. The half-life (T1/2) is relatively short and amounts to 2-3 hours.
After a 5-day course of topotecan, the total amount of topotecan and its metabolites excreted from the body ranged from 71% to 76% of the administered intravenous dose. Approximately 51% is excreted through the kidneys as topotecan, 3% as the N-demethylated metabolite of topotecan, 18% of topotecan and 1.7% of the N-demethylated metabolite of topotecan are excreted through the intestines. In general, less than 7% of topotecan is excreted as a metabolite (N-demethylated topotecan metabolite) through the kidneys and intestines (range 4% to 9%). Concentrations of topotecan O-glucuronide and N-demethylated topotecan O-glucuronide in urine are less than 2% of the administered dose.
When topotecan is administered in combination with cisplatin (cisplatin on day 1, topotecan on days 1-5), the clearance of topotecan decreases on day 5 compared to the first day (19.1 l/h/m2 compared to 21.3 l/h/m2, respectively).
In population studies, coadministration of granisetron, ondansetron, morphine or glucocorticosteroids did not have a significant effect on the pharmacokinetics of topotecan.
With repeated daily intravenous administration, topotecan accumulates in the body in minimal quantities or does not accumulate at all, and there is no data on changes in pharmacokinetics with repeated administration.
Pharmacokinetics in special clinical situations
In a population-based study of IV topotecan, a number of factors, including age, body weight and the presence of ascites, did not have a significant effect on clearance.
Children
The pharmacokinetics of topotecan given as a 30-minute infusion over 5 days was assessed in two studies. One study included a dose range of 1.4 mg/m2 to 2.4 mg/m2 in children 2 to 12 years (n=18), adolescents 12 to 16 years (n=9), and young adults 16 to 21 years (n=9) with difficult-to-treat solid tumors. The second study included a dose range from 2.0 mg/m2 to 5.2 mg/m2 in children (n=8), adolescents (n=3) and young adults (n=3) with leukemia.
In both studies, there was no obvious difference in the pharmacokinetic parameters of topotecan among different age groups, or depending on the disease (solid tumor or leukemia). These data are limited enough to allow definitive conclusions to be drawn.
Renal dysfunction
In patients with impaired renal function (creatinine clearance (CC) 41-60 ml/min), plasma clearance of intravenously administered topotecan was reduced to approximately 67% of the value in the control group. Vd decreases slightly and thus T1/2 increases by only 14%. In patients with moderate renal impairment (creatinine clearance 20-39 ml/min), plasma clearance of topotecan is reduced to 34% of the control value. Vd decreases by approximately 25%, which leads to an increase in average T1/2 from 1.9 hours to 4.9 hours.
Liver dysfunction
In patients with impaired liver function (serum bilirubin from 1.5 mg/dL to 10 mg/dL), the plasma clearance of topotecan lactone after IV administration is reduced to approximately 67% of the value in the control group, and the total plasma clearance is by 10% compared to the control group. T1/2 of topotecan increases by approximately 30%, while no significant changes in Vd are observed.
Special instructions
Special instructions
Treatment with topotecan should be carried out under the supervision of a specialist experienced in working with anticancer drugs.
The hematological toxicity of topotecan depends on its dose; it is necessary to regularly conduct detailed blood tests to determine the concentration of hemoglobin, hematocrit, counting the number of leukocytes, neutrophils and platelets.
When combining topotecan with other cytotoxic drugs, it is necessary to adjust its dose.
If severe neutropenia develops, careful monitoring is necessary for timely diagnosis of infectious complications.
As with other cytotoxic drugs, topotecan can cause severe myelosuppression, leading in some cases to severe infectious complications, including sepsis and associated death.
Neutropenia induced by topotecan treatment may cause neutropenic colitis. During clinical studies of topotecan, cases of this complication with a fatal outcome were reported. In patients with fever, neutropenia in combination with abdominal pain in the projection of the colon, the possibility of developing neutropenic colitis should be considered.
During treatment with topotecan, cases of IPD (including fatalities) have been reported.
Patients with IPD, pulmonary fibrosis, a history of lung cancer, as well as patients who have undergone chest irradiation, taken pneumotoxic drugs and/or colony-stimulating factors are at high risk of developing this complication.
Patients should be monitored for symptoms of IPD (eg, cough, fever, dyspnea, and/or hypoxia) and if new IPD is confirmed, topotecan should be discontinued.
Topotecan monotherapy and the use of topotecan in combination with cisplatin are often associated with the development of clinically significant thrombocytopenia. This should be taken into account when prescribing topotecan, for example, if the patient is at risk of developing bleeding from the tumor.
If severe thrombocytopenia occurs, extreme caution is required when performing invasive procedures, regular examination of the skin and mucous membranes, as well as secretions (to identify signs of bleeding).
It is expected that patients with low general somatic status have a lower response to treatment, and in this category of patients the incidence of complications such as fever, infectious diseases and sepsis increases. It is important to carefully assess the patient’s general somatic status during therapy in order to promptly detect its deterioration.
There is insufficient experience with the use of topotecan in patients with severe renal impairment (creatinine clearance less than 20 ml/min) or severe liver impairment (serum bilirubin > 10 mg/dl) due to cirrhosis. The use of topotecan is not recommended in this group of patients.
In a small number of patients with impaired liver function (serum bilirubin values between 1.5 and 10 mg/dL), topotecan was administered intravenously at a dose of 1.5 mg/m2 for five days every 3 weeks. In this group of patients, a decrease in the clearance of topotecan was observed. However, the data are insufficient to establish recommendations for dosage adjustments for this group of patients.
Women of reproductive age and men should use reliable methods of contraception during topotecan therapy.
Reproductive toxicity of topotecan was not observed in animal studies. However, like other cytotoxic drugs, topotecan is genotoxic and its effect on fertility, including male fertility, cannot be ruled out.
When working with the drug, it is necessary to follow generally accepted rules for handling cytotoxic drugs. If the drug accidentally comes into contact with the skin or eyes, rinse them with plenty of water.
Influence on the ability to drive vehicles and machinery
The general clinical condition of the patient and the possible development of adverse events (especially increased fatigue and weakness) should be taken into account when assessing the ability to drive vehicles and operate machinery that requires quick reaction.
Active ingredient
Active ingredient
Topotecan
Composition
Composition
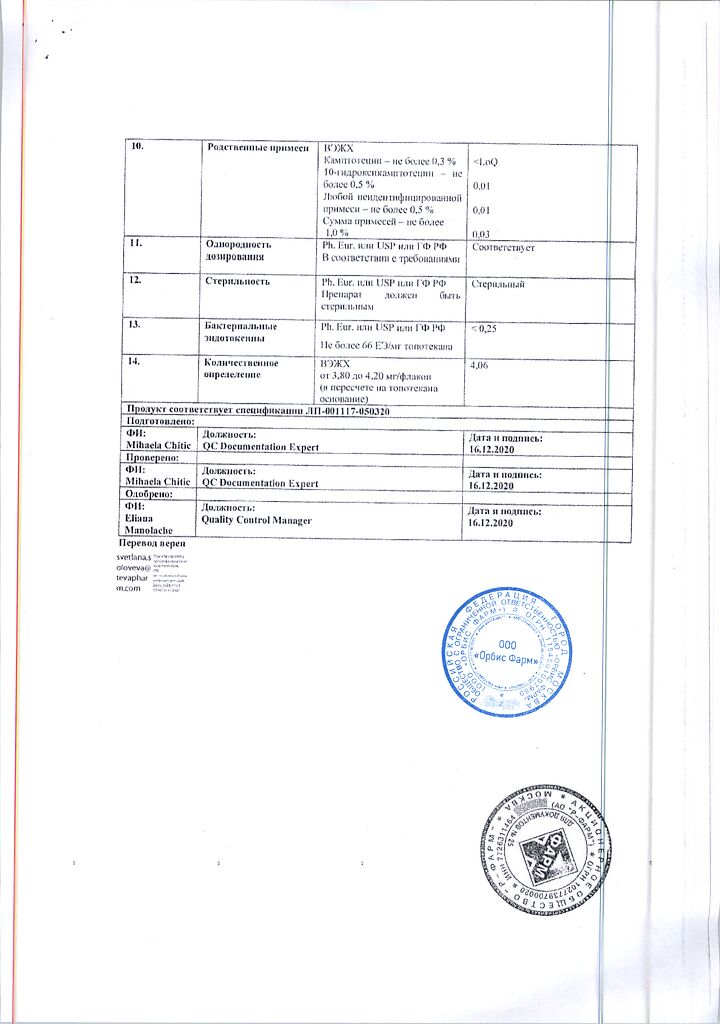
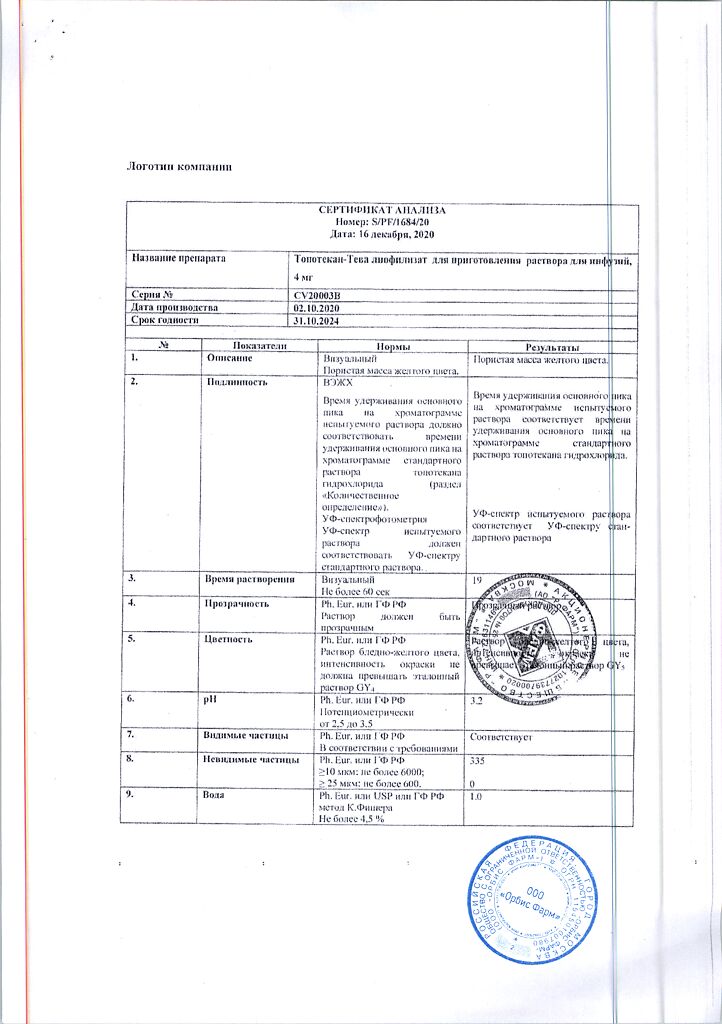
Active ingredient: topotecan hydrochloride 4.35 mg in terms of topotecan base 4.00 mg;
Excipients: mannitol 48.00 mg, tartaric acid 20.00 mg, sodium hydroxide (5 M solution) to pH 3.0 ± 0.2 (2.8-3.2).
Pregnancy
Pregnancy
Pregnancy
Preclinical studies have shown that topotecan has toxic effects on the fetus and embryo. Like other cytotoxic drugs, when used by pregnant women, Topotecan-Teva can have a negative effect on the fetus, and is therefore contraindicated for use during pregnancy.
Reliable methods of contraception should be used during drug therapy. If pregnancy occurs, you should immediately inform your doctor.
Breastfeeding period
The use of the drug is contraindicated during breastfeeding.
Contraindications
Contraindications
– pronounced suppression of bone marrow function (the number of neutrophils is less than 1.5 * 109 / l, platelets – less than 100 * 109 / l);
— anemia (Hb below 9 g/dl);
– pregnancy and breastfeeding;
– children’s age (lack of sufficient application experience);
– hypersensitivity to topotecan or other components included in the drug.
Side Effects
Side Effects
Long-term use does not increase the toxic effect of the drug. No serious manifestations of cardiotoxicity, neurotoxicity, or organ toxicity were noted.
The adverse reactions presented below are listed according to the damage to organs and organ systems, as well as by frequency of occurrence.
The incidence of adverse events was classified as follows: very common (≥1/10), common (≥1/100, <1/10), uncommon (≥1/1000, <1/100), rare (≥1/10,000, <1/1000), very rare (<1/10,000, including isolated cases), frequency unknown (cannot be estimated based on available data).
Frequency categories were formed mainly on the basis of clinical studies of topotecan.
Blood and lymphatic system disorders:
Very often – febrile neutropenia, neutropenia, thrombocytopenia, anemia1, leukopenia; often – pancytopenia; frequency unknown – bleeding, pronounced and hidden bleeding caused by thrombocytopenia.
Nervous system disorders:
Common: myalgia.
Immune system disorders:
Often – hypersensitivity reactions, including rash.
Disorders of the respiratory system, chest and mediastinal organs:
Rarely – interstitial lung disease (ILD).
Gastrointestinal disorders:
Very often – nausea, vomiting and diarrhea2, constipation, abdominal pain3, stomatitis, anorexia (including severe).
Often – hyperbilirubinemia.
Very rare: inflammation of the intestines (colitis).
Frequency unknown: intestinal obstruction.
Disorders of the skin and subcutaneous tissues:
Very often – alopecia.
Allergic reactions:
Very common: anaphylactoid reactions.
Rarely: urticaria, difficulty breathing.
Very rare: angioedema.
Not known: skin rash (including erythematous, maculopapular, urticaria, dermatitis, erythema bullosa).
General disorders and disorders at the injection site:
Very often – increased body temperature, asthenia, increased fatigue, infections.
Often – weakness, sepsis.
Very rarely – extravasation: hematoma or hyperemia of the skin at the injection site (during extravasation) (reactions associated with extravasation were mild and, as a rule, did not require specific treatment).
Frequency unknown: shortness of breath.
1 – cases of moderate to severe anemia (grades 3 and 4 – hemoglobin concentration less than 8.0 g/dL) occurred in 25% of cases (12% of courses). The median time to the onset of moderate and severe anemia is day 12, with a median duration of seven days of therapy. In 46% of treatment courses accompanied by the development of moderate and severe anemia, the duration of anemia was more than seven days. 30% of patients received red blood cell transfusion (in 13% of courses). Erythropoietin was administered to 10% of patients (in 8% of topotecan therapy courses).
2 – with intravenous administration of topotecan, diarrhea in patients over 65 years of age occurred in 10% of cases, these reactions were noted, including severe ones.
3 – cases of neutropenic colitis, including cases with a fatal outcome, were regarded as complications of drug-induced neutropenia.
There is insufficient data on the cause-and-effect relationship between the following adverse events and the use of topotecan: arthralgia, increased activity of “liver” transaminases, pain (all over the body, in the skeletal bones, in the chest), headache, neuropathy, skin itching.
Interaction
Interaction
As with other myelosuppressive cytotoxic drugs, myelosuppression is increased when topotecan is used in combination with other cytotoxic agents (eg, paclitaxel or etoposide), requiring dose reduction.
However, when topotecan is used in combination with platinum drugs (for example, cisplatin or carboplatin), there is a clear dependence of the interaction between the drugs on the sequence of their administration, i.e. depends on whether the platinum drug is administered on the 1st or 5th day of topotecan use. If platinum drugs are prescribed on day 1 of topotecan administration, reduced doses of each drug should be used compared to doses when platinum drugs are prescribed on day 5.
With intravenous administration of topotecan to 13 patients with ovarian cancer (0.75 mg/m2/day for 5 consecutive days) and cisplatin (60 mg/m2/day on day 1), the average clearance of topotecan in blood plasma on the 5th day of therapy was slightly lower than on the 1st day. As a result, systemic exposure of total topotecan, as assessed by AUC and maximum concentration Cmax, on day 5 of therapy was higher by 12% (95% confidence interval (CI): 2% to 24%) and 23% (CI: 7% to 63%), respectively. There are no data on pharmacokinetic interactions after administration of topotecan (0.75 mg/m2/day for 3 consecutive days) and cisplatin (50 mg/m2/day on day 1) in patients with cervical cancer.
Topotecan does not inhibit isoenzymes of the cytochrome P450 system. Combined use with ondansetron, granisetron, morphine or glucocorticosteroids (infusions are carried out through different systems or a different route of administration is used) does not have a significant effect on the pharmacokinetic parameters of topotecan administered intravenously.
Topotecan is a substrate for both breast cancer resistance protein BCRP (ABCG2) and ABCB1 (P-glycoprotein). Elacridar has significantly less effect on the pharmacokinetics of topotecan administered intravenously than when administered orally.
Overdose
Overdose
Symptoms
Cases of overdose have been reported in patients during therapy with topotecan in the lyophilisate dosage form for solution for infusion (overdose up to 10 times the recommended dose). Observed signs and symptoms of overdose are consistent with known adverse reactions associated with topotecan. The main complication from overdose is inhibition of bone marrow hematopoiesis and mucositis. In addition, cases of increased liver enzyme activity have been reported after overdose.
Treatment
There is no known antidote for topotecan overdose. Further treatment should be carried out as clinically indicated or as recommended by the national poison control center, if available.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C in the original packaging (bottle in a cardboard box).
Keep out of the reach of children!
Shelf life
Shelf life
4 years.
Do not use after expiration date.
Manufacturer
Manufacturer
S.K. Sindan-Pharma S.R.L., Romania
Additional information
| Shelf life | 4 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not higher than 25 ° C in the original package (bottle in carton box). Keep out of reach of children! |
| Manufacturer | S.C. Sindan-Pharma S.R.L., Romania |
| Medication form | lyophilizate |
| Brand | S.C. Sindan-Pharma S.R.L. |
Other forms…
Related products
Buy Topotecan-Teva, lyophilizate 4 mg with delivery to USA, UK, Europe and over 120 other countries.