No products in the cart.
Termicon, spray 1% 15 ml
€12.03 €10.02
Description
Pharmacotherapeutic group.
Antifungal agent
ATC code D01AE15.
Pharmacological properties.
A topical antifungal agent with a broad spectrum of antifungal activity. At low concentrations terbinafine has fungicidal effect against dermatophytes (Trychophyton rubrum, T. mentagrophytes, T. verrucosum, T. violaceum, T. tonsurans, Microsporum canis, Epidermophyton floccosum), molds, certain dimorphs (Pityrosporum orbiculare) and yeasts (mainly Сandida albicans). Activity against yeast fungi, depending on their species, can be fungicidal or fungistatic.
Terbinafine specifically alters the early stage of sterol biosynthesis that occurs in fungi. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes cell death of the fungus. The action of terbinafine is carried out by inhibiting the enzyme squaleneepoxidase located on the cell membrane of the fungus.
Terbinafine has no effect on the cytochrome P450 system in humans and therefore on the metabolism of hormones or other drugs.
Pharmacokinetics. When applied topically, absorption is less than 5%, has little systemic effect.
Indications
Indications
Prevention and treatment of fungal skin infections, including:
– mycoses of the feet (“fungus” of the foot),
– inguinal athlete’s foot,
– fungal infections of smooth body skin caused by dermatophytes (trichophytosis, microsporia, epidermophytosis, rubrophytosis).
Tinea versicolor caused by dimorphic fungi.
Diaper rash caused by mold fungi.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group.
Antifungal agent
ATX code D01AE15.
Pharmacological properties.
An antifungal drug for topical use with a wide spectrum of antifungal activity. In low concentrations, terbinafine has a fungicidal effect against dermatophytes (Trychophyton rubrum, T. mentagrophytes, T. verrucosum, T. violaceum, T. tonsurans, Microsporum canis, Epidermophyton floccosum), molds, certain dimorphic (Pityrosporum orbiculare) and yeast fungi (mainly Candida albicans). Activity against yeast fungi, depending on their type, can be fungicidal or fungistatic.
Terbinafine specifically alters the early stage of sterol biosynthesis occurring in fungi. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Terbinafine acts by inhibiting the enzyme squalene epoxidase located on the cell membrane of the fungus.
Terbinafine does not affect the cytochrome P450 system in humans and, accordingly, the metabolism of hormones or other drugs.
Pharmacokinetics. At
local application absorption is less than 5%, has a slight systemic effect
action.
Special instructions
Special instructions
A decrease in the severity of clinical manifestations is usually observed in the first days of treatment. In case of irregular treatment or its premature termination, there is a risk of recurrence of infection. If after a week of treatment there are no signs of improvement, the diagnosis should be verified.
Care should be taken when applying Thermikon® spray to damaged areas of the skin, as alcohol may cause irritation.
Thermikon® spray is intended for external use only. Avoid contact with eyes as it may cause irritation. If the drug accidentally gets into the eyes, they should be immediately rinsed with running water, and if persistent irritation develops, you should consult a doctor.
If the drug was accidentally introduced into the respiratory tract during inhalation, then if any symptoms appear and especially if they persist, you should consult a doctor.
If allergic reactions develop, the drug must be discontinued.
Impact on the ability to drive vehicles and machinery
Data about
the effect of the drug on the ability to drive vehicles or
does not work with other mechanisms.
Active ingredient
Active ingredient
Terbinafine
Composition
Composition
Composition for one cylinder (bottle).
Active substance:
terbinafine hydrochloride – 0.15 g or 0.30 g;
Excipients:
ethanol (rectified ethyl alcohol) – 5.625 g or 11.25 g,
propylene glycol – 0.30 g or 0.60 g,
poloxamer – 0.15 g or 0.30 g,
purified water – 8.775 g or 17.55 g.
Pregnancy
Pregnancy
In experimental studies, the teratogenic properties of terbinafine were not identified. To date, no malformations have been reported with the use of Thermikon®. However, since clinical experience with Thermikon® in pregnant women is very limited, it should be used only for strict indications.
Terbinafine is excreted in breast milk. However, when a nursing mother uses Thermikon® spray, a small amount of the active substance is absorbed through the skin, so adverse effects on the baby are unlikely.
Contraindications
Contraindications
Hypersensitivity to terbinafine or to the excipients included in the drug, children under 18 years of age.
With caution: liver and/or renal failure, alcoholism, suppression of bone marrow hematopoiesis, tumors, metabolic diseases, occlusive vascular diseases of the extremities.
Side Effects
Side Effects
Redness, itching or burning sensation may appear at the sites where the drug is applied. Allergic reactions.
Overdose
Overdose
No cases of drug overdose have been reported. If Thermikon® spray is accidentally taken orally, you can expect the same side effects to develop as with an overdose of Thermikon® tablets
(headache, nausea, epigastric pain and dizziness). The content of ethyl alcohol in the preparation should also be taken into account.
Treatment: activated carbon, if necessary, symptomatic maintenance therapy.
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C. Do not freeze. Store in
out of the reach of children.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
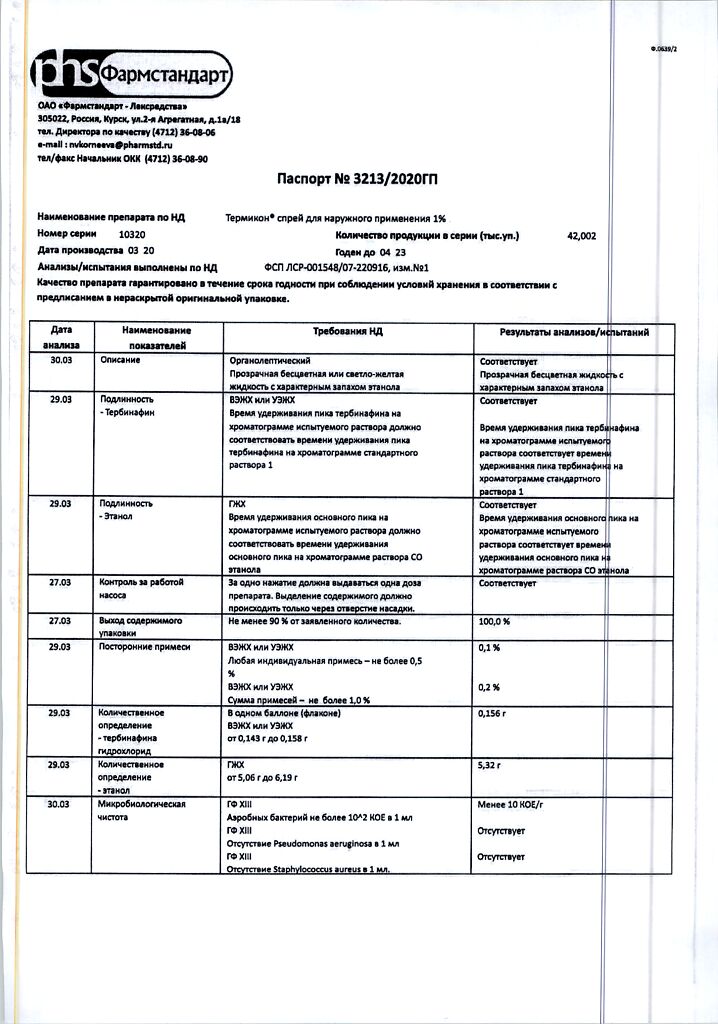
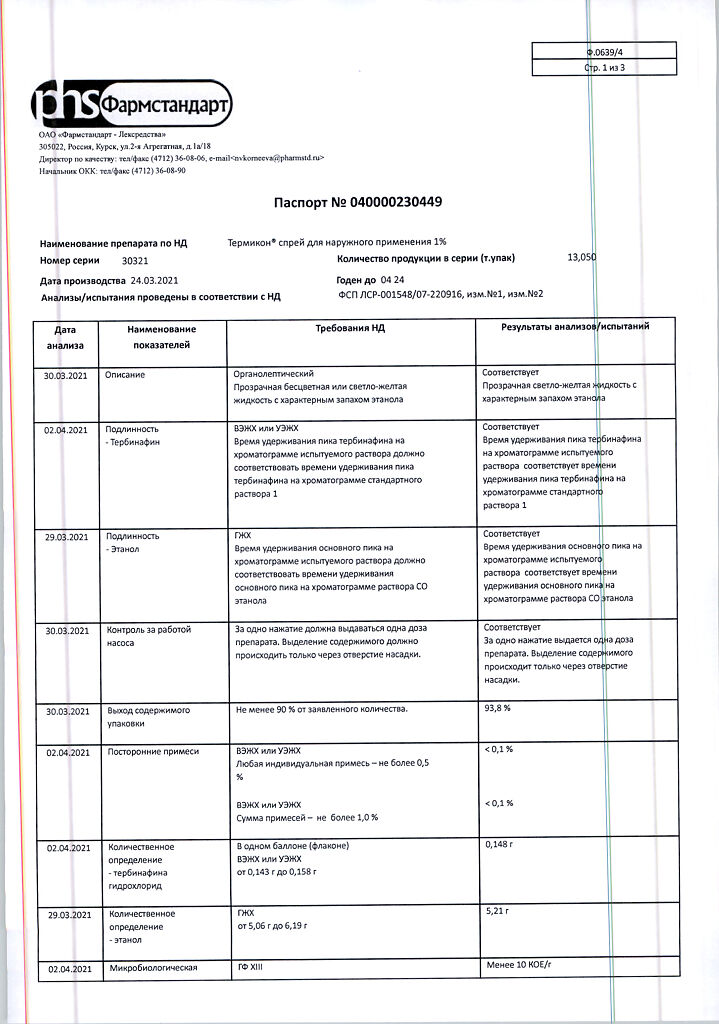
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C. Do not freeze. Keep out of reach of children. |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | topical spray |
| Brand | Pharmstandard-Leksredstva |
Other forms…
Related products
Buy Termicon, spray 1% 15 ml with delivery to USA, UK, Europe and over 120 other countries.