No products in the cart.
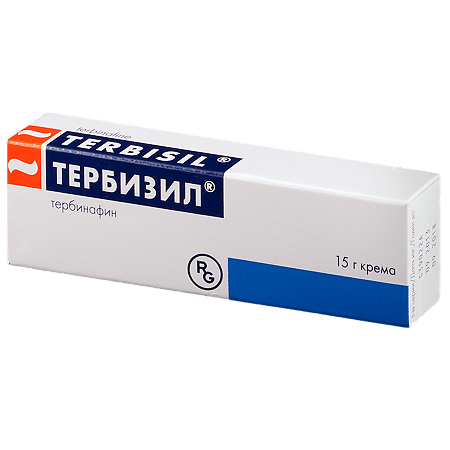
Terbisil, cream 1% 15 g
€13.47 €11.79
Description
Pharmacodynamics
Antifungal drug.
Terbinafine is an allylamine derivative. It has a wide spectrum of antifungal action. In therapeutic concentrations terbinafine has fungicidal action against dermatophytes, yeast-like fungi and some dimorphic fungi. Activity against yeast fungi, depending on their species, may be fungicidal or fungistatic.
Terbinafine specifically inhibits the early stage of sterol biosynthesis in the fungal cell by inhibiting the enzyme squalene epoxidase in the cell membrane of the fungus. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes cell death of the fungus. Squalene epoxidase is not part of the cytochrome P 450 system, so terbinafine has no effect on the metabolism of hormones or other drugs.
When applied externally, terbinafine is active against: dermatophytes – Trichophyton. Microsporum. Epidermophyton; yeast-like fungi of the genus Candida, such as: Candida albicans.
When applied externally, terbinafine is also active against: Pityrosporum orbiculare (Malassezia furfur).
Pharmacokinetics
Absorption
Less than 5% of the dose is absorbed when the cream is applied topically.
Distribution
The binding to plasma proteins is 99%. When applied externally, it quickly penetrates through the dermal layer of the skin and accumulates in the lipophilic stratum corneum. Terbinafine also penetrates into the secretion of sebaceous glands, which leads to high concentrations in hair follicles and nails.
Terbinafine is excreted with breast milk.
Metabolism and excretion
Terbinafine is metabolized in the liver to form inactive metabolites. Most of the inactive metabolites (71%) are excreted in the urine, a smaller portion (22%) in the feces.
The T 1/2 is 11 to 17 h. There is no evidence of cumulation of the drug in the body.
Pharmacokinetics in special clinical cases
In patients with impaired hepatic or renal function the elimination rate of terbinafine from the body may be reduced.
Indications
Indications
Fungal infections of the skin caused by dermatophytes, such as: Trichophyton, including: Trichophyton rubrum. Trichophyton mentagrophytes. Trichophyton verrucosum. Trichophyton violaceum. Microsporum canis. Epidermophyton floccosum.
Skin infections caused by yeast-like fungi, mainly of the genus Candida (for example, Candida albicans).
Versicolor versicolor (Pityriasis versicolor), caused by Pityrosporum orbiculare (Malassezia furfur).
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Antifungal drug.
Terbinafine is an allylamine derivative. Has a wide spectrum of antifungal action. At therapeutic concentrations, terbinafine has a fungicidal effect against dermatophytes, yeast-like fungi and some dimorphic fungi. Activity against yeast fungi, depending on their type, can be fungicidal or fungistatic.
Terbinafine specifically inhibits the early stage of sterol biosynthesis in the fungal cell by inhibiting the enzyme squalene epoxidase in the fungal cell membrane. This leads to ergosterol deficiency and intracellular accumulation of squalene, which causes the death of the fungal cell. Squalene epoxidase does not belong to the cytochrome P 450 system, therefore terbinafine does not affect the metabolism of hormones or other drugs.
When applied externally, terbinafine is active against: dermatophytes – Trichophyton. Microsporum. Epidermophyton; yeast-like fungi of the genus Candida, for example: Candida albicans.
When used externally, terbinafine is also active against: Pityrosporum orbiculare (Malassezia furfur).
Pharmacokinetics
Suction
When the cream is applied topically, less than 5% of the dose is absorbed.
Distribution
Plasma protein binding is 99%. When applied externally, it quickly penetrates the dermal layer of the skin and accumulates in the lipophilic stratum corneum. Terbinafine also penetrates the secretions of the sebaceous glands, which leads to the creation of high concentrations in the hair follicles and nails.
Terbinafine is excreted in breast milk.
Metabolism and excretion
Terbinafine is metabolized in the liver to form inactive metabolites. Most of the inactive metabolites (71%) are excreted in the urine, a smaller part (22%) is excreted in the feces.
T1/2 ranges from 11 to 17 hours. There is no evidence of drug accumulation in the body.
Pharmacokinetics in special clinical situations
In patients with impaired liver or kidney function, the rate of elimination of terbinafine from the body may be reduced.
Special instructions
Special instructions
It should be borne in mind that if during treatment with Terbizil the patient experiences liver dysfunction (weakness, nausea of unknown etiology, lack of appetite, jaundice, darkening of urine or lightening of stool), the drug should be discontinued.
During treatment, it is necessary to monitor the level of liver transaminases.
Terbizil in cream form is intended for external use only. Avoid contact of the cream with the mucous membrane of the eyes. If the cream gets into your eyes, rinse them with plenty of water and, if necessary, consult a doctor.
When treating with Terbizil, general hygiene rules should be followed to prevent the possibility of re-infection (through underwear or shoes).
It should be borne in mind that irregular use or premature discontinuation of the drug leads to relapse of the disease.
If after 2 weeks of using the drug in the form of a cream, no improvement is observed, the diagnosis and sensitivity of the pathogen to terbinafine should be clarified.
Use in children
Prescribing the cream to children is possible only after a careful assessment of the benefit-risk ratio.
Use for liver dysfunction
In patients with severe liver dysfunction, the dose of the drug should be halved.
Use for renal impairment
In patients with severe renal impairment (creatinine clearance less than 50 ml/min or serum creatinine concentration more than 300 µmol/l), the dose of the drug should be halved.
Active ingredient
Active ingredient
Terbinafine
Composition
Composition
1 g of cream for external use contains:
Active substance:
terbinafine 10 mg.
Excipients:
sodium hydroxide,
benzyl alcohol,
sorbitan monostearate,
cetyl palmitate,
cetyl alcohol,
cetostearyl alcohol (cetyl alcohol 60%, stearyl alcohol 40%),
polysorbate 60,
isopropyl myristate,
purified water.
Pregnancy
Pregnancy
Due to the fact that there is no data on the safety of terbinafine in pregnant women, Terbizil is used in this category of patients only in cases where the expected positive effect outweighs the potential risk.
Terbinafine is excreted in breast milk, so use of the drug during lactation is contraindicated.
Contraindications
Contraindications
Hypersensitivity to the components of the drug. Pregnancy and lactation (breastfeeding).
Side Effects
Side Effects
Local reactions: when using the cream, rarely – itching, burning sensation or hyperemia at the site of application (requiring cessation of therapy).
Interaction
Interaction
Drug interactions with Terbizil in cream form have not been described.
Overdose
Overdose
There is no data on an overdose of terbinafine when applied topically.
Storage conditions
Storage conditions
At 15–30 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Gedeon Richter, Hungary
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At 15-30 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | exterior cream |
| Brand | Gedeon Richter |
Related products
Buy Terbisil, cream 1% 15 g with delivery to USA, UK, Europe and over 120 other countries.