No products in the cart.
Description
Pharmacokinetics Distribution The pharmacokinetics of docetaxel is dose-dependent and follows a three-phase pharmacokinetic model with T1/2 in α-, β- and γ-phases of 4 min, 36 min and 11.1 h, respectively. After a one-hour infusion of docetaxel at a dose of 100 mg/m, the mean plasma Cmax values of docetaxel were 3.7 μg/mL with a corresponding AUC value of 4.6 μg×h/mL. The average Vd value in equilibrium is 113 l. Binding of docetaxel to plasma proteins is more than 95%. Metabolism and excretion The average value of total clearance in equilibrium is 21 l/h/m. Values of total clearance of docetaxel varied by approximately 50% in different patients. After oxidation of the tert-butyl ether group with the participation of P450 isoenzymes, docetaxel is excreted within 7 days by the kidneys, in the urine (6% of the administered dose) and through the gastrointestinal tract, with feces (75% of the administered dose). About 80% of the administered dose of docetaxel is excreted in the feces within 48 hours as metabolites (the main inactive metabolite and three less significant inactive metabolites) and in very small amounts – unchanged. Pharmacokinetics in special clinical cases Pharmacokinetics of docetaxel is independent of patient age and sex. In mild hepatic dysfunction (ALT and ACT activity not more than 1.5 their GHN in combination with ALP activity not more than 2.5 GHN) total clearance of docetaxel is decreased by 27% on average. In mild to moderate fluid retention, docetaxel clearance is unchanged; there is no information about its clearance in severe fluid retention. When combined, docetaxel does not affect doxorubicin clearance or the plasma concentration of doxorubicinol (a metabolite of doxorubicin). Pharmacokinetic parameters of docetaxel, doxorubicin and cyclophosphamide did not change with their concomitant use. Capecitabine does not affect the pharmacokinetics of docetaxel (Cmax, AUC), and docetaxel, in turn, does not affect the pharmacokinetics of capecitabine and the most important metabolite of capecitabine (5′-DFUR). Clearance of docetaxel in combination therapy with cisplatin does not change compared to its clearance in monotherapy. The pharmacokinetic profile of cisplatin administered shortly after infusion of docetaxel did not differ from that of cisplatin alone. Prednisone did not affect the pharmacokinetics of docetaxel administered after standard premedication with dexamethasone. Combination therapy with docetaxel, cisplatin and fluorouracil does not change their pharmacokinetic parameters. In children pharmacokinetic parameters during docetaxel monotherapy and docetaxel therapy in combination with cisplatin and fluorouracil were similar to those of adults. Pharmacodynamics An antitumor drug of plant origin (from the taxoid group). It accumulates tubulin in microtubules, prevents their decomposition, which disrupts the process of tumor cell division. Docetaxel persists for a long time in cells, where its concentration reaches high values. Besides, docetaxel shows activity against some, though not all, cells producing excessive amounts of P-glycoprotein (P-gP), encoded by the gene of multiple resistance to chemotherapeutic drugs. In vivo, docetaxel has a wide range of activity against mouse tumors and transplanted human tumor cells. The efficacy of docetaxel has been proven in breast cancer, non-small cell lung cancer, ovarian cancer, hormone-resistant prostate cancer, gastric cancer, head and neck cancer.
Indications
Indications
operable breast cancer BC (Taxotere® in combination with doxorubicin and cyclophosphamide, adjuvant chemotherapy):
Active ingredient
Active ingredient
Composition
Composition
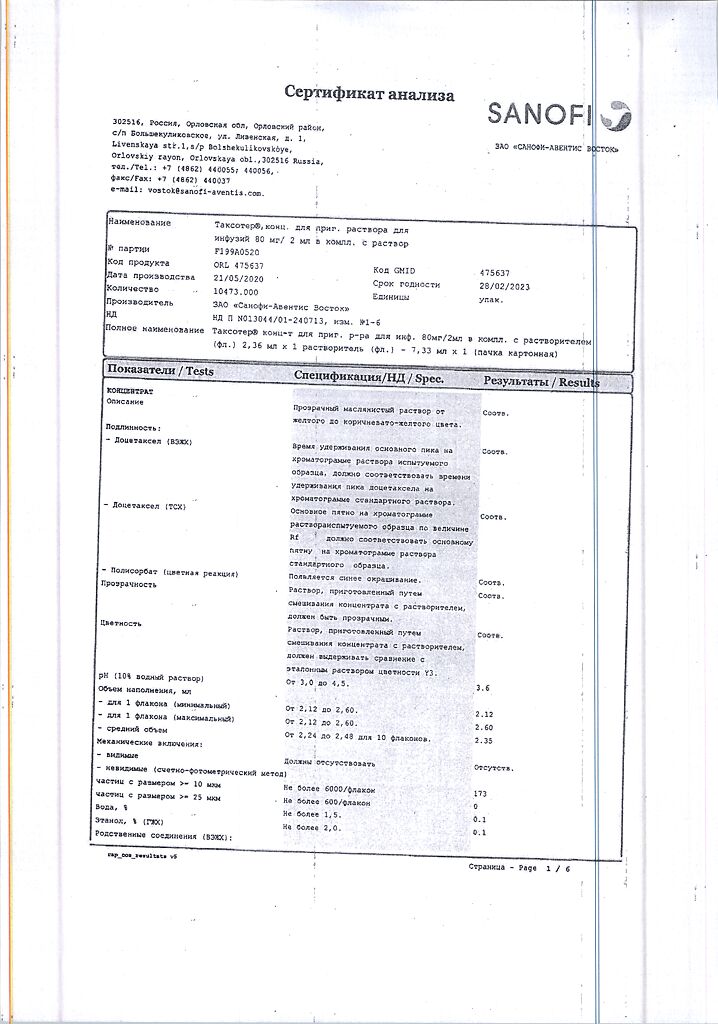
2 ml of the concentrate contain:
Active ingredients: docetaxel (in the form of docetaxel trihydrate) 80 mg.
Excipients: polysorbate 80 – up to 2.0 ml.
Solvent: (13% ethanol solution in d/I water) ethanol 95% (V/V) – 764.4 mg, d/I water – up to 6.0 ml.
The bottle contains 2.36 ml of concentrate. In the carton 1 vial, complete with solvent (7.33 ml).
How to take, the dosage
How to take, the dosage
Treatment with Taxotere® should only be performed under the supervision of a physician experienced in antitumor chemotherapy in a specialized hospital setting.
In order to prevent hypersensitivity reactions and to reduce fluid retention, all patients (excluding prostate cancer patients) should be premedicated with GCS, such as dexamethasone at a dose of 16 mg/day (8 mg twice daily) orally for 3 days, starting 1 day before the administration of Taxotere®.
In patients with prostate cancer receiving concomitant treatment with prednisone or prednisolone, dexamethasone is premedicated at doses of 8 mg 12, 3 and 1 hour before the start of Taxotere®. Prophylactic administration of G-CSF is recommended to reduce the risk of hematologic complications.
Taxotere® is administered by IV drip for 1 hour once every 3 weeks.
Breast cancer
In adjuvant therapy of operable breast cancer with regional lymph node involvement and operable breast cancer without regional lymph node involvement, the recommended dose of Taxotere® is 75 mg/msup>2 1 h after administration of doxorubicin (50 mg/m2) and cyclophosphamide (500 mg/m2) every 3 weeks (TAC regimen). A total of 6 cycles.
In locally advanced or metastatic breast cancer, docetaxel 75 mg/m2 as first-line therapy administered in combination with doxorubicin 50 mg/m2; as 2nd-line therapy, the recommended dose of docetaxel for monotherapy is 100 mg/m2.
When combining Taxotere® plus trastuzumab, the recommended dose of Taxotere® is 100 mg/m2 every 3 weeks with a weekly infusion of trastuzumab. The initial infusion of docetaxel is given the day after the first trastuzumab dose. Subsequent doses of docetaxel are administered immediately after the end of the trastuzumab infusion (if the previous trastuzumab dose is well tolerated). Doses and routes of administration of trastuzumab are specified in the instructions for medical use of trastuzumab.
In combination with capecitabine, the recommended dose of docetaxel is 75 mg/m2 every 3 weeks and capecitabine is 1250 mg/m2 orally 2 times/day (within 30 minutes after meals) for 2 weeks, followed by a 1-week rest period. To calculate the dose of capecitabine according to body surface area, see the instructions for medical use of capecitabine.
Non-small cell lung cancer
In patients who have not previously received chemotherapy, the following regimen is recommended: docetaxel 75 mg/m2, immediately followed by cisplatin at a dose of 75 mg/m2 for 30-60 minutes. For treatment after ineffectiveness of platinum-based chemotherapy, docetaxel monotherapy at a dose of 75 mg/m2 is recommended.
Metastatic ovarian cancer
For 2nd-line therapy of ovarian cancer, Taxotere® is recommended as monotherapy at a dose of 100 mg/m2 every 3 weeks.
Prostate cancer
The recommended dose of Taxotere® is 75 mg/m2 once every 3 weeks. Prednisone or prednisolone is administered long-term at 5 mg orally 2 times daily.
Stomach cancer
The recommended dose of Taxotere® is 75 mg/m2 as a one-hour infusion followed by an infusion of cisplatin at a dose of 75 mg/m2 for 1-3 hours (both drugs only on the first day of each chemotherapy cycle). Upon completion of cisplatin administration, a 24-hour infusion of fluorouracil at a dose of 750 mg/m2/day for 5 days. The treatment is repeated every 3 weeks. Premedication with anti-emetics and appropriate hydration for cisplatin administration should be performed. G-CSF administration is indicated to reduce the risk of hematologic toxicity for prophylactic purposes.
Head and Neck Cancer
Premedication with anti-emetics and appropriate hydration (before and after cisplatin administration) should be performed. Neutropenic infections should be prevented. All patients who received treatment regimens containing Taxotere® were prophylactically treated with antibiotics.
Induction chemotherapy followed by radiation therapy. For induction therapy for locally advanced inoperable squamous cell cancer of the head and neck, the recommended dose of Taxotere® is 75 mg/m2 as a one-hour intravenous infusion followed by cisplatin at a dose of 75 mg/m2 for 1 hour (both drugs are administered only on the first day of each cycle of chemotherapy). This is followed by a continuous infusion of fluorouracil at a dose of 750 mg/m2/day for 5 days. This regimen is repeated every 3 weeks for 4 cycles. After chemotherapy, patients should receive radiation therapy.
Induction chemotherapy followed by chemoradiation therapy. For induction therapy of locally advanced squamous cell cancer of the head and neck (technically unresectable, with low probability of surgical cure, or when organ preservation is decided), the recommended dose of Taxotere® is 75 mg/m2 as a one-hour IV infusion followed by a 0.5-3-hour cisplatin infusion at a dose of 100 mg/m2 (both drugs are administered only on the first day of each chemotherapy cycle) followed by a continuous infusion of fluorouracil at a dose of 1000 mg/m2/day from day 1 to day 4. This treatment regimen is repeated every 3 weeks, for a total of 3 cycles. After chemotherapy, patients should receive chemoradiotherapy. For information on dose adjustments of cisplatin and fluorouracil, the instructions for use of these drugs should be used.
Dose adjustment
General principles
Taxotere® should be administered when the peripheral blood neutrophil count is â¥1500/μL. In cases of febrile neutropenia, neutrophil counts < 500/µl lasting more than one week, marked or cumulative (increased with repeated injections) skin reactions, or marked peripheral neuropathy on therapy with docetaxel, its dose should be decreased from 100 mg/msup>2 to 75 mg/m2 and/or from 75 mg/m2 to 60 mg/m2. If such reactions persist at the 60 mg/m2 dose of docetaxel, treatment with the drug should be discontinued.
Adjuvant therapy for breast cancer
Breast cancer patients receiving adjuvant therapy with Taxotere® in combination with doxorubicin and cyclophosphamide (TAC regimen) should receive G-CSF for primary prevention. Patients who have experienced febrile neutropenia or neutropenic infection should have the dose of Taxotere® reduced to 60 mg/m2 in all subsequent cycles. In patients who have developed grade 3 or 4 stomatitis, the dose of docetaxel should be reduced to 60 mg/m2.
Taxotere® in combination with cisplatin
In patients who initially received docetaxel at a dose of 75 mg/m2 in combination with cisplatin and in whom the platelet count decreased to 25,000/μL in the previous cycle, or in patients who developed febrile neutropenia or in patients with severe non-hematologic toxicity, the dose of docetaxel in subsequent cycles should be reduced to 65 mg/m2.
The instructions for the medical use of cisplatin should be used to adjust the dose of cisplatin.
Taxotere® in combination with capecitabine
For dose adjustment of capecitabine in combination with Taxotere® , refer to the medical instructions for use of capecitabine.
In the first occurrence of grade 2 toxicity that persists by the start of the next Taxotere®/capecitabine cycle, the next treatment cycle may be delayed until toxicity decreases to grade 0-1 severity, with 100% of the original dose administered during the next treatment cycle.
In patients with recurrent development of severity 2 toxicity or the first development of severity 3 toxicity at any time during the cycle, treatment is delayed until toxicity decreases to 0-1 severity, then treatment with Taxotere® is resumed at a dose of 55 mg/m2.
In any subsequent onset of toxicity or onset of any grade 4 toxicity, Taxotere® should be discontinued.
Taxotere® in combination with trastuzumab
The instructions for medical use of trastuzumab should be used to adjust the dose of trastuzumab.
Taxotere® in combination with cisplatin and fluorouracil
Patients receiving Taxotere® in combination with cisplatin and fluorouracil should receive antiemetics and adequate hydration according to current accepted guidelines. G-CSF should be used to reduce the risk of complicated neutropenia.
If febrile neutropenia, prolonged neutropenia, or neutropenic infection occurs despite G-CSF administration, the dose of Taxotere® should be reduced from 75 to 60 mg/m2. If episodes of complicated neutropenia subsequently develop, it is recommended that the dose of Taxotere® be reduced from 60 mg/m2 to 45 mg/m2. If thrombocytopenia of severity 4 develops, it is recommended that the dose of Taxotere® be reduced from 75 mg/m2 to 60 mg/m2, Subsequent cycles with docetaxel are possible at neutrophil counts >1500/μL and platelet counts >100,000/μL. If these toxic manifestations persist, treatment should be discontinued.
Recommended dose adjustment for development of toxicity in patients receiving Taxotere® in combination with cisplatin and fluorouracil (FU)
Toxicity
Dose adjustment
Diarrhea 3 severity
First episode: Decrease the dose of FU by 20%
Repeated episode: reduce the dose of Taxotere® by 20%
Diarrhea 4 severity
First episode: reduce the dose of Taxotere® and FU by 20%
Repeated episode: discontinue treatment
Stomatitis/mucositis grade 3
First episode: reduce the dose of FU by 20%
Repeated episode: Discontinue only FU use in all subsequent cycles
Third episode: reduce the dose of Taxotere® by 20%
Grade 4 stomatitis/mucositis
First episode: Discontinue only the use of FU in all subsequent cycles
Repeated episode: reduce the dose of Taxotere® by 20%
For cisplatin and fluorouracil dose adjustment recommendations, use appropriate medical instructions.
In non-small cell head and neck cancer patients who have developed complicated neutropenia (including prolonged neutropenia, febrile neutropenia, or infection), G-CSF is recommended in all subsequent cycles for prophylactic purposes (eg, days 1 through 15).
Particular patient groups
Children. The safety and efficacy of Taxotere® in children has not been adequately studied. There is limited experience with Taxotere® in children. The efficacy and safety of Taxotere® in nasopharyngeal cancer in children and adolescents aged 1 month to 18 years has not yet been established. Taxotere® has not been used in children for the following indications: breast cancer, non-small cell lung cancer, prostate cancer, gastric cancer, and head and neck cancer, except for low-differentiated nasopharyngeal cancer (types I and II).
Patients in the elderly. Based on the data of population pharmacokinetic analysis, there are no specific indications for the use of Taxotere® in elderly patients. In patients aged 60 years and older, when combining Taxotere® with capecitabine it is recommended to reduce the dose of capecitabine by 25% (in accordance with the instructions for medical use of capecitabine).
Patients with hepatic impairment. Based on pharmacokinetic data obtained for Taxotere® at a monotherapy dose of 100 mg/m2, in patients with ALT and/or ACT activity > 1.5 VGN or ALP activity > 2.5 VGN, the recommended dose of Taxotere® is 75 mg/m2. In patients with elevated blood bilirubin ( > 1 VGN) and/or elevated ALT and ACT activity ( > 3.5 VGN) combined with elevated ALP activity (> 6 VGN), no dose reduction can be recommended and docetaxel should not be used without a strict indication.
The combination of Taxotere® with cisplatin and fluorouracil in the treatment of gastric cancer has not been used in patients with elevated ALT and/or ACT activity ( > 1.5 VGN) in combination with elevated ALP activity ( > 2.5 VGN) and elevated blood bilirubin ( > 1 VGN); no dose reduction can be recommended in such patients and docetaxel should not be used without a strong indication.
There are currently no data regarding the use of Taxotere® in combination with other drugs in patients with hepatic impairment.
Patients with impaired renal function. There are no data on the use of docetaxel in patients with severe renal impairment.
Preparation of infusion solution
Taxotere® concentrate for infusion solution (20 mg/1 ml, 80 mg/4 ml and 160 mg/8 ml) containing concentrate and solvent in one vial should not be used together with Taxotere® forms of drug in 2 vials (concentrate and solvent).
Taxotere® infusion solution concentrate (20 mg/1 ml, 80 mg/4 ml and 160 mg/8 ml) containing concentrate and solvent in one vial does not require pre-dilution with solvent and is ready to be added to the infusion solution.
Each vial is for single use and must be used immediately.
If vials are stored in refrigerator, the required number of Taxotere® vials with concentrate for preparation of infusion solution should be kept at room temperature (not exceeding 25°C) for 5 min before using them to prepare the infusion solution.
The required volume of Taxotere® 20 mg/1 mL concentrate for preparation of infusion solution, according to the required dose, under aseptic conditions, is extracted from vials using one graduated syringe connected to a 21G needle and injected into an infusion bag or bottle containing 250 mL of 5% dextrose solution or 09% sodium chloride solution (injection is performed by injecting the entire required dose into the infusion bottle once). If the required dose of docetaxel exceeds 190 mg, larger volume infusion vessels should be used to ensure that the concentration of docetaxel does not exceed 0.74 mg/ml.
The contents of the infusion bag or vial should be stirred by slowly inverting them. Infusion of the resulting solution should be carried out no later than 6 h after preparation (including 1 h of administration) when stored at room temperature and under normal light conditions. After preparation of the infusion solution under aseptic conditions, its physical and chemical stability has been demonstrated for 48 h when stored at 2° to 8°C in a non-PVC container.
The Taxotere® 20 mg/1 ml, 80 mg/4 ml and 160 mg/8 ml infusion solution concentrate and infusion solution, like any other parenteral preparations, should be inspected before administration: if there is sediment, they should not be administered and should be discarded.
The remains of the drug and all materials used to dilute and administer it should be disposed of according to standard guidelines.
Interaction
Interaction
In vitro studies have shown that the biotransformation of the drug may change with concomitant use of substances inducing or inhibiting cytochrome CYP3A isoenzymes or metabolizing (competitive inhibition) with cytochrome CYP3A, such as cyclosporine, terfenadine, ketoconazole, erythromycin and troleandomycin. Because of this caution should be exercised when prescribing such drugs concomitantly, given the possibility of pronounced interaction.
When docetaxel is coadministered with CYP3A4 isoenzyme inhibitors such as antifungal drugs from imidazole group (ketoconazole, itraconazole) and protease inhibitors (ritonavir) caution is necessary.
Studies conducted in patients receiving docetaxel and ketoconazole concomitantly showed that the clearance of docetaxel decreased by 50%, apparently due to the fact that the main metabolic pathway of docetaxel is its metabolism with participation of CYP3A4 isoenzyme. In this case, even when using docetaxel at lower doses, its tolerability may be impaired.
The pharmacokinetics of docetaxel in the presence of prednisolone has been studied in patients with metastatic prostate cancer. Although docetaxel is metabolized with participation of CYP3A4 isoenzyme and prednisolone is an inducer of CYP3A4 isoenzyme, no statistically significant effect of prednisolone on docetaxel pharmacokinetics was observed.
Docetaxel is characterized by a high degree of binding to plasma proteins (>95%). In vitro drugs that firmly bind to plasma proteins, such as erythromycin, diphenhydramine, propranolol, propafenone, phenytoin, salicylates, sulfamethoxazole and sodium valproate, do not impair the binding of docetaxel to plasma proteins. Dexamethasone also does not affect the degree of binding of docetaxel to plasma proteins. Docetaxel has no effect on the binding of digitoxin to plasma proteins.
The pharmacokinetics of docetaxel, doxorubicin and cyclophosphamide were not altered when used together.
There are reports of interactions between docetaxel and carboplatin. When using a combination of carboplatin and docetaxel, the clearance of carboplatin is increased by 50% compared with carboplatin monotherapy.
Special Instructions
Special Instructions
Contraindications
Contraindications
Side effects
Side effects
Overdose
Overdose
There are few reports of overdose.
Symptoms: suppression of bone marrow function, peripheral neurotoxicity and mucositis (inflammation of mucous membranes).
Treatment: Currently, the antidote to docetaxel is not known. In case of overdose, hospitalization in a specialized department and close monitoring of vital organs is required. G-CSF should be administered as soon as possible. If necessary, symptomatic therapy should be carried out.
Similarities
Similarities
Additional information
| Shelf life | 24 months |
|---|---|
| Conditions of storage | In a light-protected place at 2-25 °C |
| Manufacturer | Sanofi-Aventis Deutschland GmbH, Germany |
| Medication form | concentrate for preparation of infusion solution |
| Brand | Sanofi-Aventis Deutschland GmbH |
Related products
Buy Taxotere, 80 mg/2 ml concentrate 2.36 ml with delivery to USA, UK, Europe and over 120 other countries.