No products in the cart.
Spasmalgon, tablets 50 pcs
€16.57 €14.96
Description
Spasmalgon is a spasmoanalgesic of combined composition.
Methamisole sodium is a derivative of pyrazolone. It has analgesic, antipyretic and anti-inflammatory effects, the mechanism of which is associated with inhibition of prostaglandin synthesis.
Pitophenone hydrochloride is a myotropic antispasmodic; it has a direct effect on the smooth muscles of internal organs and causes their relaxation.
Phenpiperinia bromide, being a m-cholinoblocker, has an additional relaxing effect on the smooth muscles.
The combination of the three components of the drug leads to a mutual enhancement of their pharmacological action.
Special instructions
Special instructions
The drug Spazmalgon® contains lactose monohydrate, wheat starch and sodium
If you have celiac disease (congenital gluten intolerance), then Spazmalgon® can be taken. Patients with a wheat allergy (wheat allergy and celiac disease are not the same thing) should not take this drug.
Do not take Spazmalgon® if you have lactose intolerance or lactase deficiency (the enzyme that breaks down lactose).
This medicine contains less than 1 mmol (23 mg) sodium per tablet, which is essentially sodium-free.
Driving vehicles and working with machinery
The active ingredient of the drug, fenpiverinium bromide, can cause dizziness and impaired accommodation (the ability of the eyes to see at close and far distances). Patients who drive vehicles or operate machinery should be warned about the possible side effects of the drug. Activities requiring increased attention should be discontinued until side effects disappear.
Active ingredient
Active ingredient
Metamizole sodium, Pitophenone, Fenpiverinium bromide
Composition
Composition
The active ingredients are:
metamizole sodium
pitofenone hydrochloride
fenpiverinium bromide
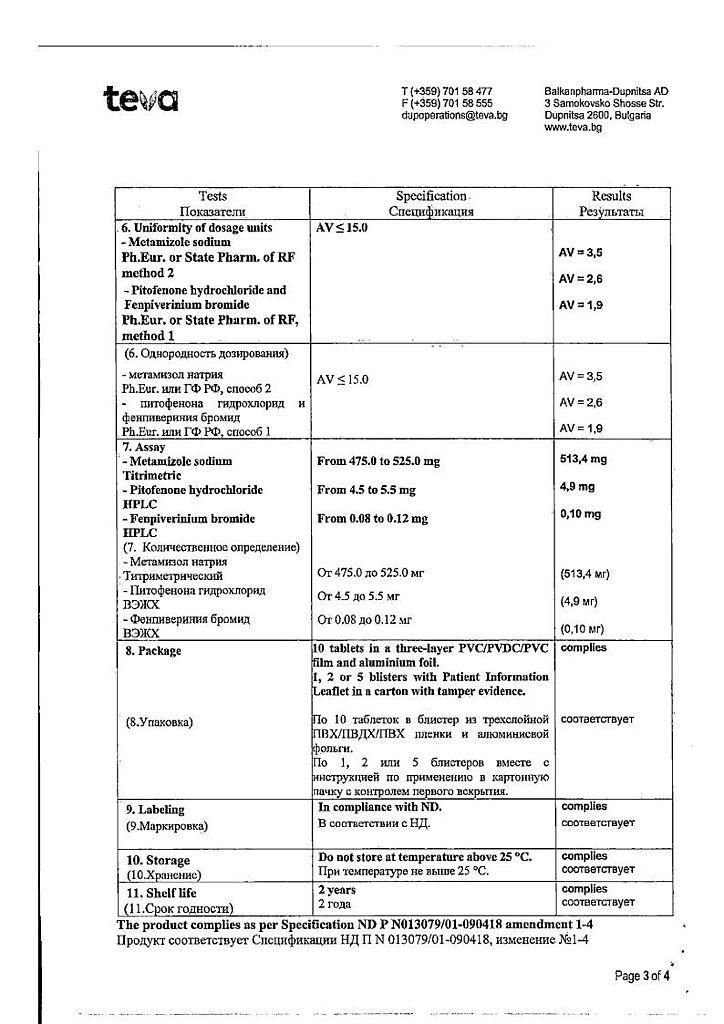
Each tablet contains: metamizole sodium 500 mg, pitofenone hydrochloride 5 mg, fenpiverinium bromide 0.1 mg
Other ingredients (auxiliary substances) are:
Lactose monohydrate
Wheat starch
Talc (magnesium hydrosilicate)
Magnesium stearate
Gelatin
Sodium bicarbonate
Pregnancy
Pregnancy
Pregnancy
If you are planning a pregnancy, think you are pregnant, or are pregnant, do not take Spazmalgon®.
Breastfeeding
If you are breastfeeding, do not take Spazmalgon®.
Fertility
There are no data on the effect of Spazmalgon®.
Contraindications
Contraindications
allergy to metamizole sodium, pitofenone hydrochloride, fenpiverinium bromide or any other ingredients of this drug listed in paragraph 6.
allergy to other pyrazolone and pyrazolidine derivatives;
gastrointestinal obstruction and megacolon (enlargement of the colon);
atony of the bladder (difficulty urinating due to weak bladder muscles) or gallbladder (atony);
severe renal or liver failure;
severe heart disease (tachyarrhythmias, severe angina; decompensated chronic heart failure);
blood diseases with changes in cellular composition, such as agranulocytosis – a strong decrease and complete absence of the total number of white blood cells (leukocytes) in the blood; leukopenia – a decrease in the number of leukocytes in the blood; aplastic anemia;
some congenital diseases (glucose-6-phosphate dehydrogenase deficiency, porphyria);
angle-closure glaucoma (a condition characterized by a rapid increase in eye pressure);
lactose intolerance, lactase deficiency, glucose-galactose malabsorption;
prostatic hyperplasia (with clinical manifestations);
wheat allergy;
children under 8 years of age;
pregnancy or breastfeeding.
Special instructions and precautions
Before taking Spazmalgon®, consult your doctor, pharmacist, or nurse.
Spazmalgon® should be taken with caution by patients with kidney and/or liver disorders, with certain stomach diseases (achalasia – non-opening of the lower esophageal sphincter when swallowing and the absence of normal esophageal motility; gastroesophageal reflux; pyloric stenosis), enlarged prostate gland, with increased thyroid function (hyperthyroidism), a tendency to low blood pressure (hypotension), severe heart rhythm disturbances, coronary heart disease (especially with a recent myocardial infarction), late stage of congestive heart failure, chronic bronchitis and bronchospasm (due to increased density of bronchial sputum), with increased sensitivity to non-steroidal anti-inflammatory drugs and non-narcotic analgesics and other allergic reactions (allergic rhinitis, bronchial asthma).
Liver lesions
There are known cases of liver inflammation in patients taking metamizole sodium. Symptoms develop within a few days to several months after starting treatment.
Stop taking Spasmalgon® and consult your doctor if you experience symptoms of liver damage such as nausea, vomiting, fatigue, loss of appetite, dark urine, light-colored stool, yellowing of the skin or whites of the eyes, itching, rash, or pain in the upper abdomen. Your doctor will do a liver test.
Do not take Spazmalgon® if you have previously taken a medicine containing metamizole sodium and have had liver problems.
With prolonged use of the drug (more than one week), monitoring of the peripheral blood picture and the functional state of the liver is necessary.
It is possible that existing headaches may appear or worsen after prolonged treatment with analgesics (more than 3 months when taken every other day or more often). Headache caused by excessive use of analgesics should not be treated by increasing the dosage. In this case, treatment with analgesics should be discontinued after consultation with a doctor.
Metabolites (decomposition products) of metamizole sodium can cause urine to turn red, which is not clinically significant.
When used together with alcohol and substances that suppress the functions of the central nervous system, the drug may affect the mental state of patients.
Allergic reactions
If you have an allergy, be sure to tell your doctor about it.
An increased risk of developing allergic reactions to metamizole sodium, which is part of the drug, is possible if you have the following conditions:
bronchial asthma, especially in combination with polypous rhinosinusitis;
chronic urticaria;
alcohol intolerance (increased sensitivity to alcohol), against the background of which, even when taking a small amount of certain alcoholic beverages, patients experience sneezing, lacrimation and severe redness of the face. Alcohol intolerance may indicate previously unidentified aspirin asthma syndrome;
intolerance or hypersensitivity to dyes (for example, tartrazine) or preservatives (for example, benzoate).
Serious skin reactions
There have been case reports of serious skin reactions such as Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS syndrome) associated with metamizole sodium. If you develop a progressive skin rash, often with blisters or lesions on the mucous membrane, stop treatment with Spasmalgon® immediately and seek medical attention.
Under no circumstances should you resume taking Spazmalgon® if you have had any severe skin reactions in the past.
Blood count disorders
Taking Spazmalgon®, as a drug containing metamizole sodium, can affect the composition of the blood. If symptoms appear – fever, chills, pale skin, sore throat, difficulty swallowing, stomatitis, bleeding, erosive and ulcerative lesions of the oral cavity, vaginitis or proctitis (inflammation of the rectum), a decrease in the number of neutrophils in the peripheral blood – less than 1500/mm3 – you must stop treatment and consult a doctor. This condition can last at least a week, does not depend on the dose, can be severe and threaten your life. If you are receiving antibiotics, typical symptoms may be minimal.
When the platelet count decreases, there is an increased tendency to bleeding and the appearance of petechiae (rash) on the skin and mucous membranes.
Hypotension
Administration of a metamizole-containing drug may cause a decrease in blood pressure. To avoid the development of severe hypotensive reactions, monitor blood pressure and heart rate.
Alcohol
During treatment with the drug you should refrain from drinking alcohol.
Stomach ache
Do not take the drug to relieve acute abdominal pain and consult a doctor.
Drug-induced liver damage
When taking Spazmalgon®, hepatitis (liver inflammation) may occur in the period from several days to several months after the start of treatment. Immediately stop taking the drug if yellow discoloration of the skin and mucous membranes, skin rash, fever, nonspecific blood diseases appears, and consult a doctor.
After discontinuation of Spazmalgon®, these reactions disappear, however, in isolated cases, a worsening of the condition may be observed.
Do not take Spazmalgon® again if liver dysfunction has previously occurred while taking this drug, and no other causes of the disorder have been found.
Children and teenagers
Do not give this medicine to children under 8 years of age due to the possible safety risk.
Side Effects
Side Effects
Like all medicines, this medicine can cause side effects, although not all patients experience these reactions.
Stop taking Spazmalgon® and call your doctor immediately if you experience any of the following symptoms: nausea or vomiting, fatigue, loss of appetite, dark urine, light-colored stool, yellowing of the skin or whites of the eyes, itching, rash, or pain in the upper abdomen. These symptoms may be signs of liver damage.
If any of the following serious side effects occur, stop using Spasmalgon® immediately and seek medical attention:
– red-brown flat, target-shaped or round spots on the body, often with blisters in the center, peeling skin, ulcers on the mucous membrane of the mouth, throat, nose, genitals and eyes. Such serious skin rashes may be preceded by fever and a flu-like illness (Stevens-Johnson syndrome, toxic epidermal necrolysis).
– rash all over the body, high body temperature and swollen lymph nodes (DRESS syndrome or drug hypersensitivity syndrome).
There are also other undesirable reactions:
Very common – may affect more than 1 in 10 people:
visual disturbances
disturbances of accommodation
Common – may occur in no more than 1 in 10 people:
dizziness
headache
bronchospasm
Uncommon – may occur in up to 1 in 100 people:
fixed drug exanthema
feeling of heartbeat
tachycardia
heart rhythm disorder
cyanosis
arterial hypotension
hyperemia
Rare – may occur in no more than 1 person in 1000:
leukopenia (decreased number of white blood cells in the blood)
anaphylactic shock
angioedema
decreased sweating
proteinuria (protein in urine)
oliguria (decreased amount of urine excreted)
anuria (almost complete absence of urine output)
polyuria (increased urination)
interstitial nephritis (kidney disease)
red coloration of urine
difficulty urinating
renal dysfunction
Very rare – may occur in up to 1 in 10,000 people:
agranulocytosis
thrombocytopenia
anemia (hemolytic anemia, aplastic anemia)
attack of bronchial asthma (in patients with “aspirin” asthma)
Frequency unknown – the frequency of occurrence cannot be determined based on the available data:
dry mouth
nausea
vomit
pain and discomfort in the abdomen
constipation
in rare cases, bloody vomiting and intestinal bleeding
Interaction
Interaction
Tell your doctor or pharmacist if you are taking, have recently taken, or may start taking any other medications. Some medications may affect the effect of Spazmalgon® when taken together – they can increase or decrease the effect of Spazmalgon®.
It is especially important to tell your doctor what you are taking:
• H1-histamine receptor blockers, butyrophenone and phenothiazine derivatives, amantadine and quinidine. With simultaneous use, the effect of these drugs may be enhanced.
• Other non-narcotic analgesics. Concomitant use may result in mutually reinforcing toxic effects.
• Tricyclic antidepressants, oral contraceptives, allopurinol (for the treatment of gout) interfere with the metabolism of metamizole sodium in the liver and increase its toxicity.
• With simultaneous use of the drug with tricyclic antidepressants, its m-anticholinergic effect may be enhanced.
• Barbiturates (hypnotics), phenylbutazone reduce the effect of metamizole sodium.
• Concomitant use of metamizole sodium with the drugs tacrolimus, cyclosporine (taken to prevent organ transplant rejection), sertraline (taken to treat depression), valproic acid (taken to treat epilepsy and bipolar disorder), methadone (to treat drug addiction), efavirenz (taken to treat HIV/AIDS) may lead to a decrease in the concentration of the latter in the blood plasma and a decrease in their therapeutic value. effect.
• Sedatives and tranquilizers (medicines that calm the nervous system) enhance the analgesic effect of the drug.
• Chlorpromazine or other phenothiazine derivatives. Concomitant use may lead to the development of severe hyperthermia (increased body temperature).
• Radiocontrast agents, colloidal blood substitutes and penicillin should not be used during treatment with drugs containing metamizole sodium, as the risk of anaphylactic reactions increases.
• Oral hypoglycemic drugs, indirect anticoagulants, glucocorticosteroids and indomethacin. Simultaneous use may lead to an increase in their activity.
• Methotrexate. Concomitant use may lead to increased toxic effects on blood cells of the latter, especially in elderly patients.
• Thiamazole and sarcolysine. Concomitant use increases the risk of developing leukopenia (decreased white blood cells).
• Codeine, H2-histamine receptor blockers and propranolol enhance the effects of metamizole sodium.
Spazmalgon® with alcohol
Simultaneous use of Spazmalgon® with alcoholic beverages enhances the effects of ethanol.
Overdose
Overdose
In case of an overdose of the drug, the following symptoms may be observed: vomiting, a feeling of dry mouth, decreased sweating, impaired accommodation, decreased blood pressure, drowsiness, confusion, impaired liver and kidney function, convulsions.
Treatment – gastric lavage, administration of activated carbon, symptomatic therapy.
Action
Action
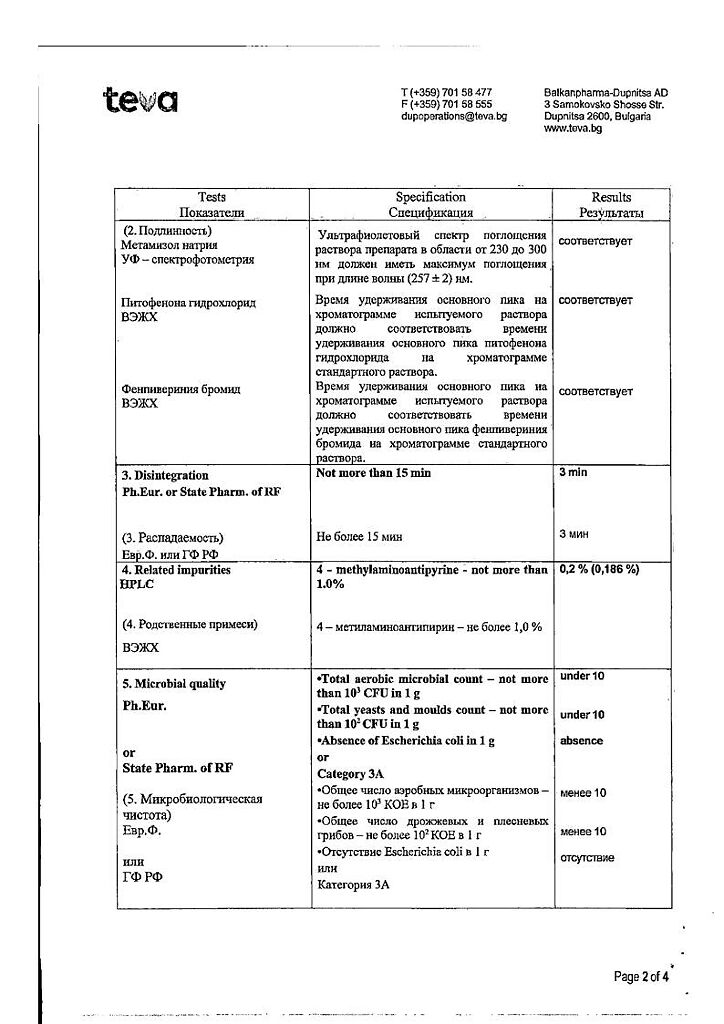
Metamizole sodium is a pyrazolone derivative that has an analgesic, antipyretic and anti-inflammatory effect. Suppresses cyclooxygenase and reduces the formation of prostaglandins from arachidonic acid.
Pitophenone hydrochloride has a direct effect on the smooth muscles of internal organs and causes its relaxation.
Phenpiverinium bromide has an additional relaxing effect on smooth muscles.
Storage conditions
Storage conditions
Keep the drug out of the reach of children and so that the child cannot see it.
Do not use the drug after the expiration date (shelf life) indicated on the package after the words “Best before:”.
The expiration date is the last day of the given month.
Store at a temperature not exceeding 25 °C.
No special precautions are required for storing the drug.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Balkanpharma – Dupnitsa AD, Bulgaria
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | Store in a dry place protected from light, at a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Balkanfarma – Dupnitsa AD, Bulgaria |
| Medication form | pills |
| Brand | Balkanfarma – Dupnitsa AD |
Other forms…
Related products
Buy Spasmalgon, tablets 50 pcs with delivery to USA, UK, Europe and over 120 other countries.