No products in the cart.
Solkovagin, 0.5 ml solution 2 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
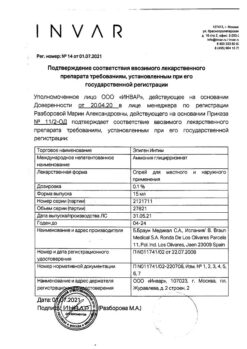
Pharmgroup:
The drug for the treatment of benign lesions of the cervix.
Pharmaceutical action:
Solkovagin is a drug for local treatment of benign lesions of superficial tissues of the cervix.
When using the drug there is a differential effect on different types of cervical epithelium.
When the drug is applied to the ectopic cylindrical epithelium (cervical ectopy and transformation zone) and subepithelial stroma (cervical erosion) their immediate devitalization and fixation in vivo occurs. On the other hand, the multi-layered cell structures of the squamous epithelium of the vaginal cervix and the vaginal mucosa, which are more resistant to the effects of Solkovagin, remain virtually intact.
Devitalization and fixation of the altered tissue occurs within minutes and is accompanied by a yellowish-white or gray staining of the tissue. This phenomenon cannot be categorized as a cauterizing effect of the acids as normally understood since the devitalized (necrotized) epithelium remains in its place during the initial phase and forms a protective layer which peels off after a few days due to the spontaneous growth of new squamous epithelial cells beneath it.
Pharmacokinetics:
The drug Solkovagin acts only at the site of application. No systemic absorption is observed.
Indications
Indications
Benign lesions of superficial tissues of the cervix: cervical ectopy, transformation zone, nabotum cysts (after dissection), polyps of the cervical canal (in the absence of endometrial pathology), postoperative granulomas.
Active ingredient
Active ingredient
Composition
Composition
1 ml of solution contains
nitric acid 70% 537 mg,
99% acetic acid 20.4 mg,
p> Oxalic acid dihydrate 58.6 mg,
Zinc nitrate hexahydrate 6 mg,
Distilled water to 1 ml
How to take, the dosage
How to take, the dosage
Solkovagin is used topically.
The procedure should only be performed by a specialist – gynecologist (using a colposcope).
In the procedure, only the affected area should be treated, avoiding contact of the solution with the skin of the external genitalia and the epithelium of the vagina.
Interaction
Interaction
Till now there have been no cases of interaction of Solkovagin with other drugs.
Special Instructions
Special Instructions
The treatment with Solkovagin is performed on an outpatient basis and does not impose any restrictions on water procedures and sexual activity.
You should avoid contact of the drug with clothing, skin and especially in the eyes.
In case of contact of Solkovagin solution with the skin of the external genitalia or the epithelium of the vagina, the area of contact with the drug should be immediately rinsed with water.
If Solkovagin solution comes into contact with the skin or eyes, immediately rinse the affected area with copious amounts of water and, if possible, with a neutralizing solution such as 1% sodium bicarbonate solution.
After the procedure, place the used cotton swab 1 cm into the vial, break off the stem, close the vial with the cap included in the package to prevent residual solution from dripping on surrounding objects, then discard in a waste container.
Contraindications
Contraindications
Malignant changes of the cervix or suspected malignization, cellular dysplasia, pregnancy, hypersensitivity to the components of the drug.
Side effects
Side effects
To date, no side effects have been noted with Solkovagin.
The treatment is painless and does not result in scarring or deformation of the cervical canal.
Overdose
Overdose
Symptoms: overuse of the drug may cause burns, which may lead to pathological changes in the cervical epithelium.
Treatment: symptomatic.
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 20 °C. |
| Manufacturer | Meda Pharma GmbH & Co. |
| Medication form | topical solution |
| Brand | #Н/Д |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Buy Solkovagin, 0.5 ml solution 2 pcs with delivery to USA, UK, Europe and over 120 other countries.