No products in the cart.
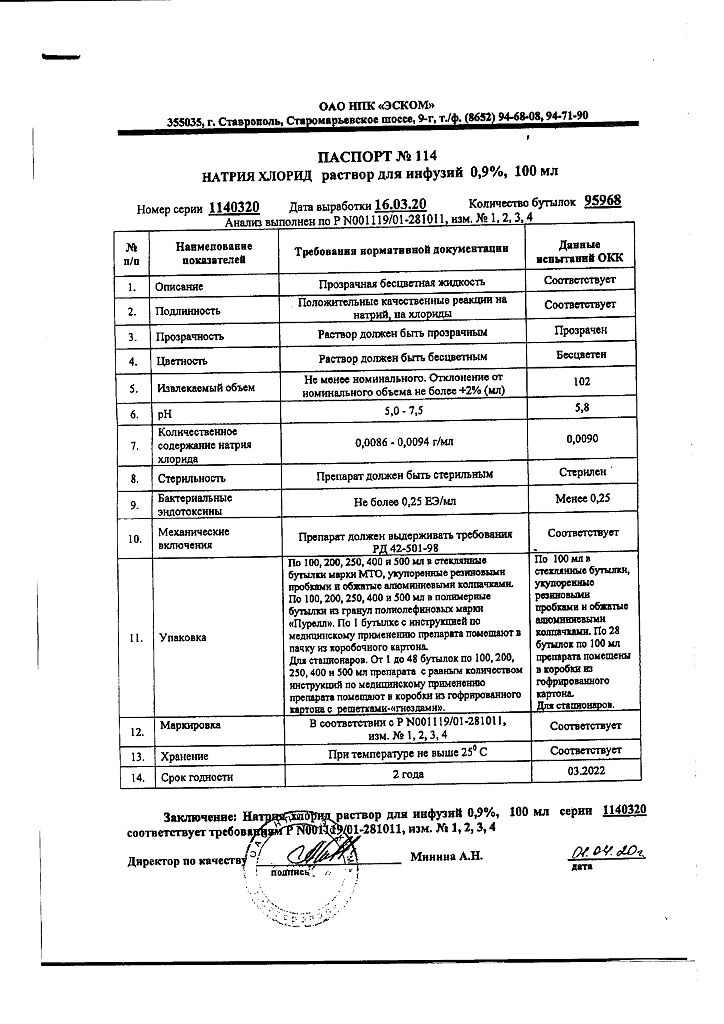
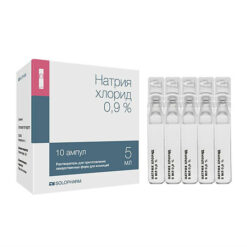
Sodium chloride, 0.9% 100 ml
€1.00
Out of stock
(E-mail when Stock is available)
Description
It has a detoxifying and rehydrating effect. It replenishes sodium deficiency in various pathological states of the body and temporarily increases the volume of fluid circulating in blood vessels.
The pharmacodynamic properties of the solution are due to the presence of sodium ions and chloride ions. A number of ions, including sodium ions, pass through the cell membrane by various transport mechanisms, among which the sodium-potassium pump (Na-K-ATPase) is important. Sodium plays an important role in signal transduction in neurons, the electrophysiological processes of the heart, as well as in metabolic processes in the kidneys.
Sodium is excreted mainly by the kidneys, however, a large amount of sodium is reabsorbed (renal reabsorption). A small amount of sodium is excreted in the feces and by perspiration.
Indications
Indications
isotonic extracellular dehydration;
hyponatremia;
dilution and dissolution of parenterally administered medicinal substances (as a base solution).
Pharmacological effect
Pharmacological effect
Has a detoxifying and rehydrating effect. Replenishes sodium deficiency in various pathological conditions of the body and temporarily increases the volume of fluid circulating in the vessels.
The pharmacodynamic properties of the solution are due to the presence of sodium and chloride ions. A number of ions, including sodium ions, penetrate the cell membrane using various transport mechanisms, among which the sodium-potassium pump (Na-K-ATPase) is of great importance. Sodium plays an important role in neuronal signal transmission, electrophysiological processes in the heart, and metabolic processes in the kidneys.
Sodium is excreted primarily by the kidneys, however, large amounts of sodium are reabsorbed (renal reabsorption). A small amount of sodium is excreted in feces and through sweating.
Special instructions
Special instructions
When performing any infusion, it is necessary to monitor the patient’s condition, clinical and biological indicators, it is especially important to evaluate plasma electrolytes. In children, sodium excretion may slow down due to immature kidney function. Therefore, in such patients, repeated infusions should be carried out only after determining the plasma sodium concentration.
Use only a clear solution, without visible inclusions, if the packaging is not damaged. Administer immediately after connecting to the infusion system.
As with all parenteral solutions, the compatibility of added substances with the solution must be determined before reconstitution.
Drugs known to be incompatible with it should not be used with sodium chloride solution 0.9%. A doctor should determine the compatibility of added medicinal substances with a 0.9% sodium chloride solution by checking for possible changes in color and/or the appearance of sediment, insoluble complexes or crystals.
Before adding, it is necessary to determine whether the substance being added is soluble and stable in water at the pH level of a 0.9% sodium chloride solution.
The temperature of the infusion solution should be 38°C.
Active ingredient
Active ingredient
Sodium chloride
Composition
Composition
100 ml of solution contains:
sodium chloride 900 mg
Contraindications
Contraindications
hypernatremia, acidosis, hyperchloremia, hypokalemia, extracellular hyperhydration;
circulatory disorders that threaten cerebral and pulmonary edema;
cerebral edema, pulmonary edema, acute left ventricular failure, concomitant administration of corticosteroids in large doses.
When adding other drugs to the solution, it is necessary to take into account contraindications to these drugs.
With caution: decompensated chronic heart failure, arterial hypertension, peripheral edema, preeclampsia, chronic renal failure (oligo-, anuria), aldosteronism and other conditions associated with sodium retention in the body.
Side Effects
Side Effects
Acidosis, overhydration, hypokalemia.
When used correctly, unwanted effects are unlikely.
When using sodium chloride solution 0.9% as a base solution (solvent) for other drugs, the likelihood of side effects is determined by the properties of these drugs. In this case, if adverse reactions occur, the administration of the solution should be suspended, the patient’s condition assessed, adequate measures taken, and the remaining solution retained for analysis, if necessary.
Interaction
Interaction
Not described.
When mixing with other drugs, it is necessary to visually monitor compatibility.
To do this, you should monitor the resulting solution for changes in its color and/or precipitation, the appearance of crystals, and insoluble complexes. It is also necessary to take into account the instructions for use of the added drugs.
Overdose
Overdose
Symptoms: nausea, vomiting, diarrhea, abdominal cramps, thirst, decreased salivation and lacrimation, sweating, fever, tachycardia, increased blood pressure, renal failure, peripheral edema, pulmonary edema, respiratory arrest, headache, dizziness, anxiety, irritability, weakness, muscle cramps and rigidity, generalized convulsions, coma and death. Excessive administration of the solution may cause hypernatremia.
Excessive intake of chloride into the body can lead to hyperchlorimic acidosis.
When used as a base solution for diluting and dissolving other drugs, symptoms and complaints with excessive administration are most often associated with the properties of the drugs administered.
In case of unintentional over-administration of the solution, treatment should be stopped and the patient’s condition assessed.
Treatment: symptomatic.
Manufacturer
Manufacturer
Eskom NPK, Russia
Additional information
| Manufacturer | Eskom NPK, Russia |
|---|---|
| Medication form | solution for infusion |
| Brand | Eskom NPK |
Other forms…
Related products
Buy Sodium chloride, 0.9% 100 ml with delivery to USA, UK, Europe and over 120 other countries.