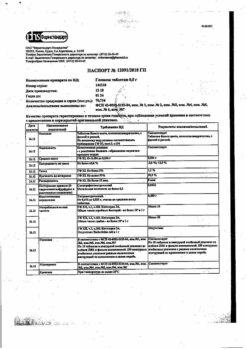
No products in the cart.
Siofor 850, 60 pcs
€4.71 €4.19
Description
Pharmacodynamics
Hypoglycemic drug of the biguanide group. It provides reduction of both basal and postprandial blood glucose concentrations. It does not stimulate insulin secretion and therefore does not lead to hypoglycemia. The action of metformin is probably based on the following mechanisms:
- reduction of glucose production in the liver due to inhibition of gluconeogenesis and glycogenolysis;
- increased muscle sensitivity to insulin and thus improved peripheral glucose uptake and utilization;
- inhibition of glucose absorption in the gut.
Metformin through its action on glycogen synthetase stimulates intracellular glycogen synthesis. Increases the transport capacity of all hitherto known membrane glucose transport proteins.
Independently of the effect on blood glucose levels, it has a favorable effect on lipid metabolism and leads to a decrease in total cholesterol, low-density cholesterol and triglycerides.
Pharmacokinetics
Intake
After oral administration, Cmax in plasma is reached after about 2.5 h and at maximum dosage does not exceed 4 mcg/ml. With food, absorption decreases and slows down slightly. Absolute bioavailability in healthy patients is approximately 50-60%.
Distribution
It is practically not bound to plasma proteins. The average Vd is 63-276 L. It accumulates in salivary glands, muscles, liver and kidneys. It penetrates the erythrocytes.
Elimation
Extracted by the kidneys unchanged. Renal clearance is >400 ml/min. T1/2 is about 6.5 h.
Pharmacokinetics in special clinical cases
In reduced renal function, metformin clearance decreases in proportion to creatinine clearance. Thus, the T1/2 is prolonged and the plasma concentration of metformin is increased.
Indications
Indications
Type 2 diabetes mellitus, especially in overweight patients with ineffective diet therapy and exercise.
It can be used as monotherapy or in combination with other oral hypoglycemic drugs and insulin.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Hypoglycemic drug from the biguanide group. Provides a decrease in both basal and postprandial blood glucose concentrations. Does not stimulate insulin secretion and therefore does not lead to hypoglycemia. The action of metformin is likely based on the following mechanisms:
decreased glucose production in the liver due to inhibition of gluconeogenesis and glycogenolysis;
increasing muscle sensitivity to insulin and, therefore, improving peripheral glucose uptake and utilization;
inhibition of glucose absorption in the intestine.
Metformin, through its action on glycogen synthetase, stimulates intracellular glycogen synthesis. Increases the transport capacity of all currently known membrane glucose transport proteins.
Regardless of the effect on blood glucose levels, it has a beneficial effect on lipid metabolism, leading to a decrease in total cholesterol, low-density cholesterol and triglycerides.
Pharmacokinetics
Suction
After oral administration, Cmax in blood plasma is reached in approximately 2.5 hours and at the maximum dosage does not exceed 4 mcg/ml. When eating, absorption decreases and slows down slightly. Absolute bioavailability in healthy patients is approximately 50-60%.
Distribution
Practically does not bind to plasma proteins. Average Vd is 63-276 hp. Accumulates in the salivary glands, muscles, liver and kidneys. Penetrates into red blood cells.
Removal
It is excreted unchanged by the kidneys. Renal clearance is >400 ml/min. T1/2 is about 6.5 hours.
Pharmacokinetics in special clinical situations
With decreased renal function, metformin clearance decreases in proportion to creatinine clearance. Thus, T1/2 is prolonged and the plasma concentration of metformin increases.
Special instructions
Special instructions
Lactic acidosis is a serious pathological condition, extremely rare, associated with the accumulation of lactic acid in the blood, which can be caused by the accumulation of metformin. The described cases of the development of lactic acidosis in patients receiving metformin were observed mainly in patients with diabetes mellitus with severe renal failure. Prevention of lactic acidosis involves identifying all associated risk factors, such as decompensated diabetes, ketosis, prolonged fasting, excessive alcohol consumption, liver failure and any condition associated with hypoxia. If the development of lactic acidosis is suspected, immediate discontinuation of the drug and emergency hospitalization are recommended.
Since metformin is excreted by the kidneys, plasma creatinine concentrations should be determined before starting treatment and regularly thereafter. Particular caution should be exercised in cases where there is a risk of impaired renal function, for example, when starting therapy with antihypertensive drugs, diuretics or NSAIDs.
Treatment with Siofor® should be temporarily replaced with therapy with other hypoglycemic drugs (for example, insulin) 48 hours before and 48 hours after an X-ray examination with intravenous administration of iodinated contrast agents.
The use of Siofor® should be discontinued 48 hours before planned surgery under general anesthesia, spinal or epidural anesthesia. Therapy should be continued after resumption of oral nutrition or no earlier than 48 hours after surgery, provided normal renal function is confirmed.
Siofor® does not replace diet and daily exercise – these types of therapy must be combined in accordance with your doctor’s recommendations. During treatment with Siofor®, all patients should adhere to a diet with even carbohydrate intake throughout the day. Overweight patients should follow a low-calorie diet.
Standard laboratory tests for patients with diabetes must be performed regularly.
Before using Siofor® in children aged 10 to 18 years, the diagnosis of type 2 diabetes mellitus should be confirmed.
During one-year controlled clinical studies, the effect of metformin on growth and development, as well as puberty in children, was not observed; data on these indicators with longer-term use are not available. In this regard, careful monitoring of relevant parameters in children receiving metformin is recommended, especially in the prepubertal period (10-12 years).
Monotherapy with Siofor® does not lead to hypoglycemia, but caution is recommended when using the drug simultaneously with insulin or sulfonylurea derivatives.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
The use of the drug Siofor® does not cause hypoglycemia, and therefore does not affect the ability to drive vehicles and maintain machinery.
When using the drug Siofor® simultaneously with other hypoglycemic drugs (sulfonylurea derivatives, insulin, repaglinide), hypoglycemic conditions may develop, so care must be taken when driving vehicles and other potentially hazardous activities that require concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Metformin
Composition
Composition
Active ingredients:
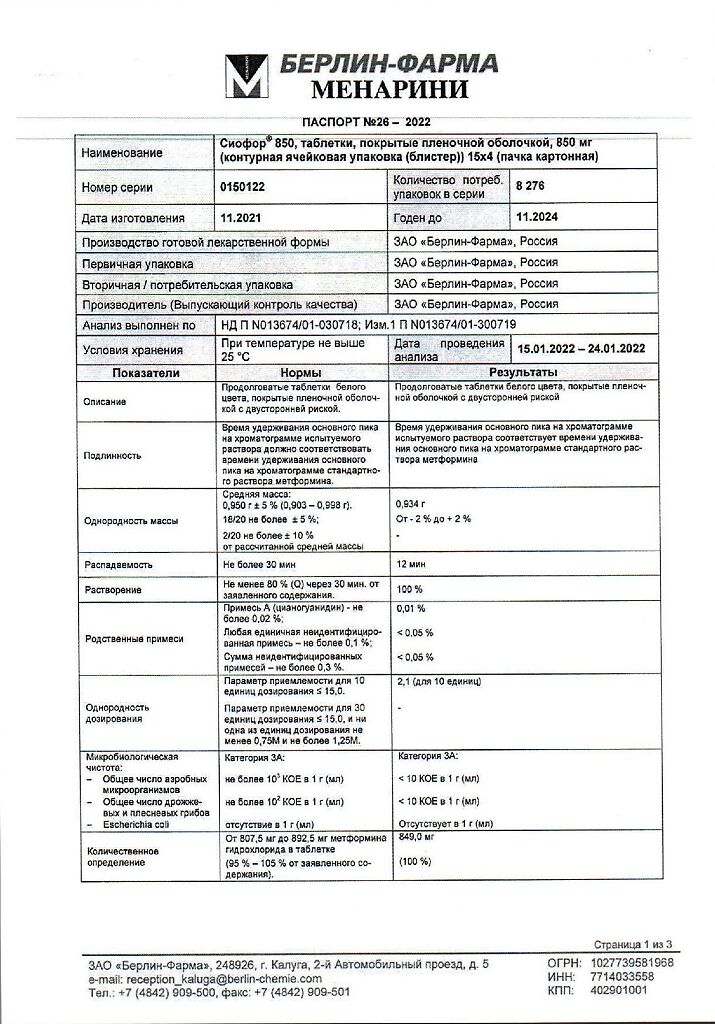
metformin hydrochloride 850 mg.
Excipients:
hypromellose – 30 mg,
povidone – 45 mg,
magnesium stearate – 5 mg.
Shell composition:
hypromellose – 10 mg,
macrogol 6000 – 2 mg,
titanium dioxide (E171) – 8 mg.
Pregnancy
Pregnancy
The drug is contraindicated for use during pregnancy and lactation (breastfeeding).
The patient should be warned about the need to notify the doctor if pregnancy occurs. When planning or becoming pregnant in a patient with type 2 diabetes mellitus, the drug should be discontinued and blood glucose levels should be normalized or brought as close to normal as possible using insulin therapy to reduce the risk of developing defects in the fetus due to the pathological effects of hyperglycemia.
Metformin passes into the milk of laboratory animals. There are no similar data for humans, so a decision should be made to stop breastfeeding or to discontinue the drug Siofor®, taking into account the need to use the drug in the mother.
Contraindications
Contraindications
Hypersensitivity to metformin or other components of the drug;
diabetic ketoacidosis, diabetic precoma;
renal failure or impaired renal function (creatinine clearance <60 ml/min);
acute conditions that can have a negative impact on kidney function (for example, dehydration, severe infectious disease);
intravascular injection of iodinated contrast agent;
acute or chronic diseases that can cause tissue hypoxia (for example, cardiac or respiratory failure, recent myocardial infarction, shock);
liver failure;
lactic acidosis (including history);
pregnancy and lactation (breastfeeding);
acute alcohol intoxication, chronic alcoholism;
following a low-calorie diet (less than 1000 kcal/day);
children under 10 years of age.
With caution: the drug should be used in children aged 10 to 12 years; in patients over 60 years of age who perform heavy physical work, which is associated with an increased risk of developing lactic acidosis.
Side Effects
Side Effects
Possible side effects when using the drug are given depending on the frequency of occurrence:
often (≥1/100, <1/10); uncommon (≥1/1000, <1/100); rare (≥1/10,000, <1/1000); very rare (<1/10,000), including isolated reports.
From the nervous system: often – taste disturbance.
From the digestive system: nausea, vomiting, “metallic” taste in the mouth, lack of appetite, diarrhea, abdominal pain. These adverse events often occur at the beginning of therapy and in most cases resolve spontaneously. To prevent the onset of symptoms, it is recommended to distribute the dose of the drug into 2-3 doses during or after meals. Gradually increasing the dose reduces the likelihood of unwanted effects from the gastrointestinal tract.
Allergic reactions: very rarely – skin reactions (for example, flushing, itching, urticaria).
Metabolic disorders: very rarely: – lactic acidosis (requires cessation of treatment). With long-term use, there is a decrease in the absorption of vitamin B12 and a decrease in its concentration in the blood plasma. This should be taken into account if the patient has megaloblastic anemia.
From the liver and biliary tract: isolated reports of reversible liver dysfunction, expressed in increased activity of hepatic transaminases, or hepatitis, which resolves after discontinuation of metformin.
Interaction
Interaction
Contraindicated combinations
Intravascular administration of iodinated contrast agents in patients with diabetes mellitus may be complicated by renal failure, as a result of which metformin accumulates and the risk of developing lactic acidosis increases. The use of the drug Siofor® should be discontinued 48 hours before the procedure and not resumed earlier than 2 days after an X-ray examination using iodine-containing contrast agents, provided that the serum creatinine concentration is normal.
Combinations not recommended
The risk of developing lactic acidosis increases with acute alcohol intoxication or simultaneous use with ethanol-containing drugs, especially in the context of dieting or malnutrition, as well as liver failure.
Combinations requiring caution
Concomitant use of metformin with danazol may lead to the development of a hyperglycemic effect. If treatment with danazol is necessary and after stopping its use, a dose adjustment of metformin is required under the control of blood glucose concentrations.
When used simultaneously with oral contraceptives, epinephrine, glucagon, thyroid hormones, phenothiazine derivatives, nicotinic acid, it is possible to increase the concentration of glucose in the blood.
Nifedipine increases absorption and Cmax in the blood plasma of metformin, prolongs its elimination.
Cationic drugs (amiloride, morphine, procainamide, quinidine, ranitidine, triamterene, vancomycin), secreted in the tubules, compete for tubular transport systems and, with long-term therapy, can increase the Cmax of metformin in the blood plasma.
Cimetidine slows down the elimination of the drug, which increases the risk of developing lactic acidosis.
Metformin reduces Cmax and T1/2 of furosemide.
Metformin may weaken the effect of indirect anticoagulants.
Glucocorticoids (for systemic and local use), beta-agonists and diuretics have hyperglycemic activity. Blood glucose concentrations should be monitored more carefully, especially at the beginning of treatment. If necessary, the dose of metformin should be adjusted during the period of simultaneous use and after discontinuation of these drugs.
ACE inhibitors and other antihypertensive drugs may lower blood glucose levels. If necessary, the dose of metformin can be adjusted.
With simultaneous use of the drug Siofor® with sulfonylurea derivatives, insulin, acarbose, and salicylates, the hypoglycemic effect may be enhanced.
Overdose
Overdose
When using metformin in doses up to 85 g, hypoglycemia was not observed.
Symptoms: with a significant overdose, lactic acidosis may develop, the symptoms of which are severe weakness, respiratory disorders, drowsiness, nausea, vomiting, diarrhea, abdominal pain, hypothermia, decreased blood pressure, reflex bradyarrhythmia. Muscle pain, confusion, and loss of consciousness may occur.
Treatment: immediate discontinuation of the drug and emergency hospitalization are recommended. The most effective method of removing lactate and metformin from the body is hemodialysis.
Storage conditions
Storage conditions
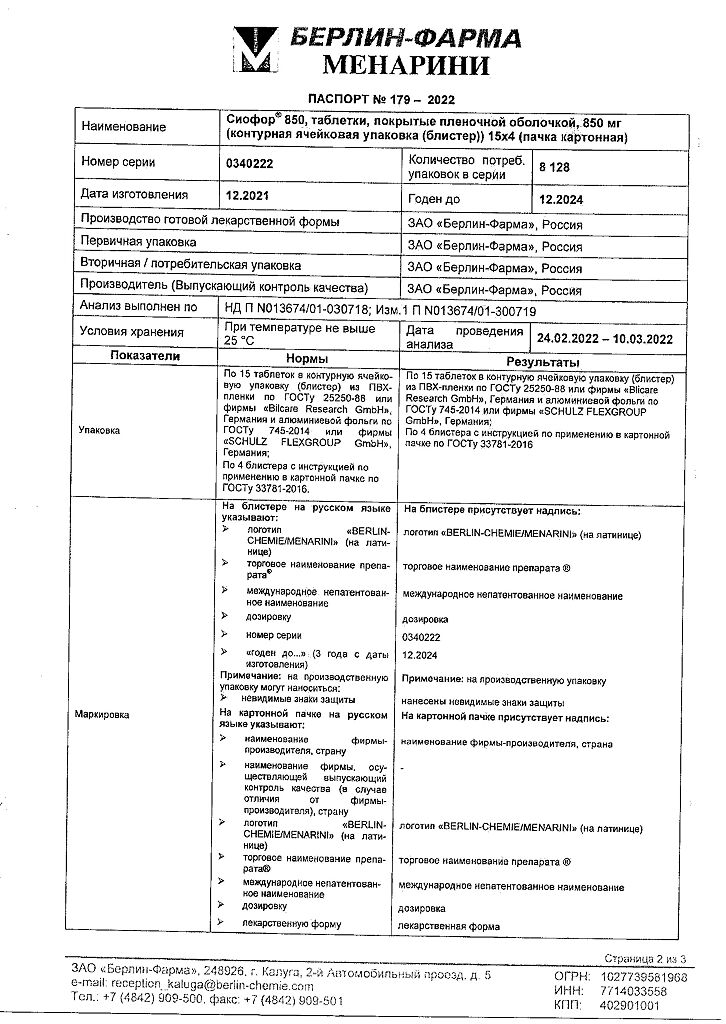
At a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Berlin-Pharma, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Berlin-Pharma, Russia |
| Medication form | pills |
| Brand | Berlin-Pharma |
Related products
Buy Siofor 850, 60 pcs with delivery to USA, UK, Europe and over 120 other countries.