No products in the cart.
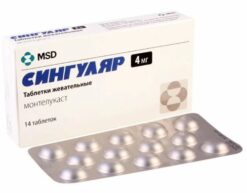
Singlon, 4 mg 28 pcs.
€39.68 €33.07
Description
Singlon is a blocker of cysteinyl leukotriene receptor CysLT1 (leukotrienes C4, D4 and E4 – mediators of chronic persistent inflammation that maintain bronchial hyperresponsiveness in bronchial asthma).
Indications
Indications
Bronchial asthma (prevention and long-term treatment), complete or incomplete combination of bronchial asthma, recurrent polyposis of the nose and paranasal sinuses and intolerance to ASA or other NSAIDs (including a history), bronchial asthma of physical exertion, allergic rhinitis.
Pharmacological effect
Pharmacological effect
Singlen is a blocker of cysteinyl leukotriene receptors CysLT1 (leukotrienes C4, D4 and E4 – mediators of chronic persistent inflammation that supports bronchial hyperreactivity in bronchial asthma).
Special instructions
Special instructions
The effectiveness of oral montelukast for the treatment of acute attacks of bronchial asthma has not been established. Therefore, oral montelukast is not recommended for the treatment of acute attacks of bronchial asthma. Patients should be instructed to always carry emergency medications to relieve asthma attacks (short-acting inhaled beta2-agonists).
You should not stop taking montelukast during an exacerbation of asthma and the need to use emergency medications (short-acting inhaled beta2-agonists) to relieve attacks.
Patients with a confirmed allergy to acetylsalicylic acid and other NSAIDs should not take these drugs during treatment with montelukast, since montelukast, while improving respiratory function in patients with allergic bronchial asthma, nevertheless cannot completely prevent bronchoconstriction caused by NSAIDs.
The dose of inhaled corticosteroids used simultaneously with montelukast can be gradually reduced under the supervision of a physician, however, an abrupt replacement of inhaled or oral corticosteroids with montelukast cannot be carried out.
Neuropsychiatric disorders have been described in patients taking montelukast. Given that these symptoms could be caused by other factors, it is unknown whether they are related to montelukast. Physicians should discuss these side effects with patients and/or their parents/guardians. Patients and/or their caregivers should be advised that if such symptoms occur, they should notify their physician.
Reducing the dose of systemic corticosteroids in patients receiving anti-asthmatic drugs, including leukotriene receptor blockers, was accompanied in rare cases by the appearance of one or more of the following reactions: eosinophilia, rash, worsening of pulmonary symptoms, cardiac complications and/or neuropathy, sometimes diagnosed as Churg-Strauss syndrome, systemic eosinophilic vasculitis. Although a causal relationship between these adverse reactions and leukotriene receptor antagonist therapy has not been established, caution and appropriate clinical monitoring should be used when reducing the dose of systemic corticosteroids in patients receiving montelukast.
Active ingredient
Active ingredient
Montelukast
Composition
Composition
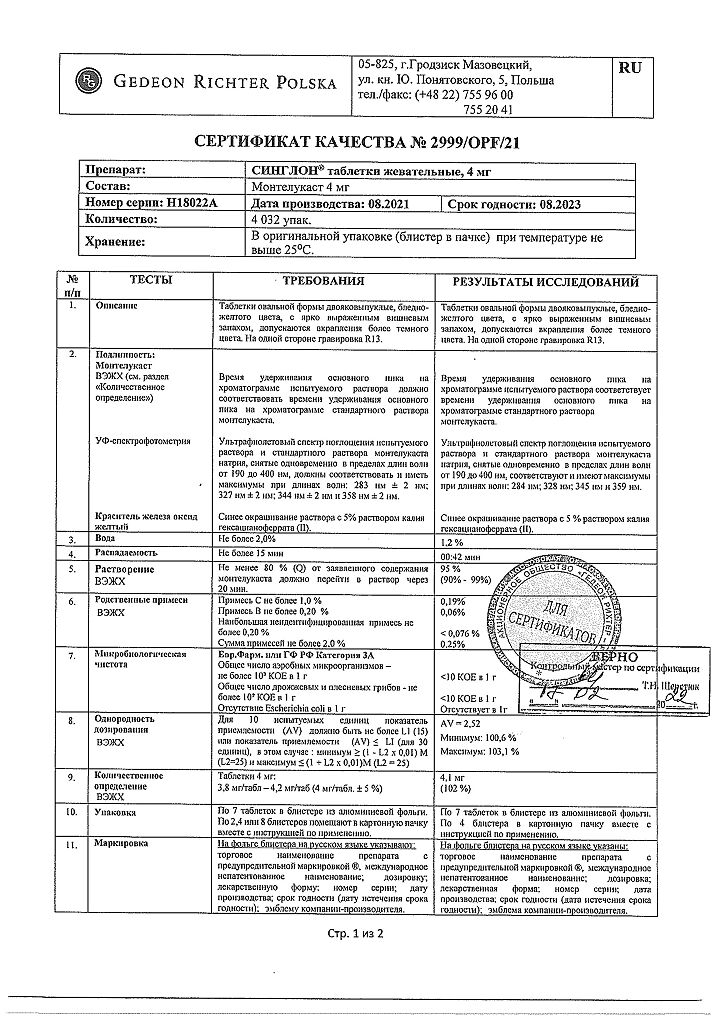
1 tablet contains montelukast 4 mg.
Contraindications
Contraindications
Hypersensitivity, children’s age (up to 6 years).
Side Effects
Side Effects
From the blood coagulation system: increased tendency to bleeding.
From the immune system: hypersensitivity reactions, incl. anaphylaxis; very rarely (
From the psyche: agitation (including aggressive behavior or hostility), anxiety, depression, disorientation, pathological dreams, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thoughts and behavior (suicidality), tremor.
From the nervous system: dizziness, drowsiness, paresthesia/hypesthesia; very rarely (<1/10,000) - convulsions.
From the cardiovascular system: tachycardia.
From the respiratory system: nosebleeds, upper respiratory tract infections.
From the digestive system: diarrhea, dyspepsia, nausea, vomiting, pancreatitis, increased activity of ALT and AST in the blood; very rarely (
From the skin and subcutaneous tissues: tendency to form hematomas, erythema nodosum, erythema multiforme, itching, rash.
Allergic reactions: angioedema, urticaria.
From the musculoskeletal system: arthralgia, myalgia, including muscle cramps.
From the body as a whole: asthenia (weakness)/fatigue, edema, pyrexia.
Interaction
Interaction
When used concomitantly with phenobarbital, the AUC of montelukast decreased by approximately 40% (no adjustment of the montelukast regimen is required).
In vitro studies have shown that montelukast inhibits the CYP2C8 isoenzyme, however, an in vivo drug interaction study between montelukast and rosiglitazone (metabolized by the CYP2C8 isoenzyme) did not confirm that montelukast inhibits the CYP2C8 isoenzyme. Therefore, in clinical practice, the effect of montelukast on CYP2C8-mediated metabolism of a number of drugs, incl. paclitaxel, rosiglitazone, repaglinide.
Montelukast is a reasonable addition to bronchodilator monotherapy if the latter do not provide adequate control of bronchial asthma. Once the therapeutic effect of montelukast treatment is achieved, a gradual reduction in the dose of bronchodilators can begin.
The use of montelukast provides an additional therapeutic effect in patients receiving inhaled corticosteroids. Once the condition has stabilized, you can begin a gradual reduction in the dose of GCS under the supervision of a physician. In some cases, complete withdrawal of inhaled corticosteroids is acceptable, but abrupt replacement of inhaled corticosteroids with montelukast is not recommended.
Overdose
Overdose
There is no specific information on the treatment of overdose with Singlon. Data on overdose symptoms when taking the drug in adult patients with bronchial asthma at a dose exceeding 200 mg/day. for 22 weeks and at a dose of 900 mg/day. not detected within 1 week.
Storage conditions
Storage conditions
Store the drug out of the reach of children, in a dry place protected from light at a temperature not exceeding 25°C.
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Gedeon Richter Poland, Poland
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | Keep the drug out of the reach of children, dry and protected from light at a temperature not exceeding 25 ° C. |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | chewable tablets |
| Brand | Gedeon Richter |
Other forms…
Related products
Buy Singlon, 4 mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.