No products in the cart.
Simvastatin Zentiva, tablets 40 mg, 28 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
SIMVACARD is a hypolipidemic drug obtained synthetically from the fermentation product of Aspergillus terreus, it is an inactive lactone, it undergoes hydrolysis in the body to form a hydroxy acid derivative. The active metabolite inhibits 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase), the enzyme that catalyzes the initial reaction of mevalonate formation from HMG-CoA. Since the conversion of HMG-CoA to mevalonate represents an early step in cholesterol synthesis, the use of simvastatin does not cause accumulation of potentially toxic sterols in the body. HMG-CoA is easily metabolized to acetyl-CoA, which is involved in many synthesis processes in the body.
It lowers plasma levels of triglycerides (TG), low-density lipoproteins (LDL), very low-density lipoproteins (VLDL) and total cholesterol (in cases of heterozygous familial and non-familial hypercholesterolemia, in mixed hyperlipidemia, when elevated cholesterol is a risk factor).
Enhances high-density lipoprotein (HDL) and decreases the ratio of LDL/HDL and total cholesterol/HDL.
The onset of the effect is 2 weeks after the start of administration, the maximum therapeutic effect is achieved after 4-6 weeks. The effect is maintained during continuation of treatment, when therapy is discontinued cholesterol content gradually returns to baseline levels.
Pharmacokinetics
The absorption of simvastatin is high. After oral administration Cmax in plasma is reached after about 1.3 – 2.4 hours and decreases by 90% after 12 hours. The binding to plasma proteins is about 95%.
Metabolized in the liver, it has the effect of first passage through the liver (it is hydrolyzed with the formation of the active derivative: beta-hydroxy acid; other active as well as inactive metabolites have also been found).
The T1/2 of active metabolites is 1.9 h. It is excreted mainly with the feces (60%) as metabolites. About 10-15% is excreted by the kidneys in the inactive form.
Indications
Indications
Hypercholesterolemia in the absence of the effect of diet therapy, combined hypercholesterolemia and triglyceridemia, CHD, prevention of myocardial infarction and stroke, atherosclerosis.
Active ingredient
Active ingredient
Composition
Composition
1 tablet contains:
The active ingredient:
simvastatin;
Auxiliary substances:
MCC;
Lactose monohydrate (milk sugar);
Pregelatinized starch (starch 1500);
colloidal silicon dioxide (aerosil);
ascorbic acid;
butyl hydroxyanisole;
stearic acid;
iron oxide dye red;
titanium dioxide.
How to take, the dosage
How to take, the dosage
Overly, once a day, in the evening, with plenty of water. The time of taking the drug should not be associated with food intake.
Before starting treatment with Simvastatin, the patient should be prescribed a standard hypocholesterolemic diet, which should be followed during the entire course of treatment.
The recommended dose of Simvastatin for treatment of hypercholesterolemia varies from 10 to 80 mg once daily in the evening. The recommended starting dose of the drug for patients with hypercholesterolemia is 10 mg. The maximum daily dose is 80 mg.
Changing (adjusting) the dose should be done at 4 week intervals. In most patients the optimal effect is achieved with doses up to 20 mg/day.
In patients with homozygous hereditary hypercholesterolemia the recommended daily dose of Simvastatin is 40 mg once daily in the evening or 80 mg in 3 doses (20 mg in the morning, 20 mg during the day and 40 mg in the evening).
When treating patients with CHD or high risk of CHD, effective doses of Simvastatin are 20-40 mg/day. Therefore, the recommended starting dose in such patients is 20 mg/day. The dose should be changed (adjusted) at 4 week intervals; if necessary, the dose can be increased to 40 mg/day. In elderly patients and patients with mild to moderately expressed renal insufficiency, there is no need to change the dosage of the drug.
In patients with CKD (creatinine Cl
In patients taking amiodarone or verapamil concomitantly with Simvastatin, the daily dose should not exceed 20 mg.
Interaction
Interaction
Cytostatics, antifungal agents (ketoconazole, itraconazole), fibrates, high-dose nicotinic acid, immunosuppressants, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, nefazodone increase the risk of myopathy.
Cyclosporine or danazole: the risk of myopathy/rhabdomyolysis is increased when cyclosporine or danazole are coadministered with high-dose simvastatin.
Other hypolipidemic agents that may cause myopathy: the risk of myopathy increases with co-administration of other hypolipidemic agents that are not potent CYP3A4 inhibitors but that may cause myopathy with monotherapy. Such as gemfibrozil and other fibrates (except fenofibrate), as well as niacin (nicotinic acid) at a dose of > 1 g/day.
Amiodarone and verapamil: the risk of myopathy increases when coadministering amiodarone or verapamil with high doses of simvastatin.
Diltiazem: the risk of myopathy increases slightly in patients receiving diltiazem concomitantly with simvastatin at a dose of 80 mg.
Simvastatin potentiates the effect of peroral anticoagulants (e.g., phenprocoumon, warfarin) and increases the risk of bleeding, which requires monitoring of blood clotting prior to treatment, and often enough during the initial period of therapy. Once a stable level of prothrombin time or International Normalized Ratio (MHO) is achieved, it should be further monitored at the intervals recommended for patients receiving anticoagulant therapy. Prothrombin time or MHO should also be monitored according to the above scheme if the dosage is changed or simvastatin is discontinued.
Therapy with simvastatin does not cause changes in prothrombin time and risk of bleeding in patients not taking anticoagulants. Increases plasma levels of digoxin.
Colestyramine and colestipol decrease bioavailability (use of simvastatin is possible 4 hours after taking these drugs, with an additive effect).
Grapefruit juice contains one or more components that inhibit CYP3A4 and may increase the plasma concentration of drugs metabolized by CYP3A4. The increase in HMG-CoA reductase inhibitor activity after consuming 250 ml of juice per day is minimal and of no clinical significance. However, consumption of a large volume of juice (more than 1 liter per day) while taking simvastatin significantly increases the level of inhibitory activity against HMG-CoA reductase in plasma. In this regard, consumption of grapefruit juice in large quantities should be avoided.
Special Instructions
Special Instructions
In the beginning of therapy with simvastatin, there may be a transient increase in liver enzymes.
Liver function tests should be performed regularly before initiating therapy and thereafter (monitor liver enzyme activity every 6 weeks for the first 3 months, then every 8 weeks for the remaining first year, and then once every six months), and a liver function test should be performed if doses are increased. If the dose is increased to 80 mg, a test should be performed every 3 months. If there is a persistent increase in transaminase activity (3 times the baseline level), simvastatin should be discontinued.
Simvastatin, like other HMG-CoA reductase inhibitors, should not be used if there is an increased risk of rhabdomyolysis and renal failure (against the background of severe acute infection, arterial hypotension, planned major surgery, trauma, severe metabolic disorders).
The withdrawal of hypolipidemic agents during pregnancy has no significant effect on the results of long-term treatment of primary hypercholesterolemia.
Due to the fact that HMG-CoA reductase inhibitors inhibit cholesterol synthesis, and cholesterol and other products of its synthesis play an essential role in fetal development, including steroid and cell membrane synthesis, simvastatin may have adverse effects on the fetus when prescribed to pregnant women (women of reproductive age should avoid conception). If pregnancy occurs during treatment, the drug should be withdrawn and the woman should be warned about the possible danger to the fetus.
The use of simvastatin is not recommended in women of childbearing age who do not use contraception.
In patients with reduced thyroid function (hypothyroidism) or in the presence of certain kidney diseases (nephrotic syndrome) when cholesterol levels increase, therapy of the underlying disease should be given first.
Simvastatin is prescribed with caution in people who abuse alcohol and/or have a history of liver disease.
The patient should be on a hypocholesterolemic diet before and during treatment.
The concomitant administration of grapefruit juice may increase the severity of side effects associated with taking simvastatin, so concomitant administration should be avoided.
Simvastatin is not indicated in cases with hypertriglyceridemia types I, IV and V.
The treatment with simvastatin may cause myopathy leading to rhabdomyolysis and renal failure. The risk of this pathology increases in patients receiving one or more of the following drugs concomitantly with simvastatin: fibrates (gemfibrozil, fenofibrate), cyclosporine, nefazadone, macrolides (erythromycin, clarithromycin), antifungal agents of azole group (ketoconazole, intraconazole) and HIV protease inhibitors (ritonavir). The risk of myopathy also increases in patients with severe renal insufficiency.
All patients starting therapy with simvastatin, as well as patients who need to increase the dose of the drug, should be warned about the possibility of myopathy and the need to seek immediate medical attention if unexplained pain, muscle soreness, sluggishness or muscle weakness occur, especially if accompanied by malaise or fever. Therapy with the drug should be stopped immediately if myopathy is diagnosed or suspected.
In order to diagnose the development of myopathy, regular measurements of CPK are recommended.
The treatment with simvastatin may increase serum CPK, which should be considered in the differential diagnosis of chest pain. The criterion for discontinuation of the drug is an increase in serum CPK more than 10 times the upper limit of normal. In patients with myalgia, myasthenia and/or marked increase of CPK activity, the drug shall be discontinued.
The drug is effective both as monotherapy and in combination with bile acid sequestrants.
If the current dose is missed, the drug should be taken as soon as possible.
If it is time to take the next dose, the dose should not be doubled.
Patients with severe renal insufficiency should have their renal function monitored.
The duration of use of the drug is determined by the attending physician individually.
Impact on driving and operating machinery
There have been no adverse effects of Simvastatin on driving and operating machinery.
Contraindications
Contraindications
Side effects
Side effects
Digestive system disorders: constipation, diarrhea, loss of appetite, flatulence, nausea, abdominal pain, pancreatitis, increased ALT, AST, GGT, ALP activity.
CNS and peripheral nervous system disorders: headache, dizziness, muscle cramps, paresthesias, peripheral neuropathy.
Cardiovascular system disorders: transient arterial hypotension is possible.
Muscular system disorders: myalgia, myopathy, rhabdomyolysis, increased CPK activity.
Allergic reactions: rarely – angioedema, lupus-like syndrome, vasculitis, thrombocytopenia, eosinophilia, increased sedimentation, arthritis, urticaria, fever, shortness of breath.
Dermatological reactions: photosensitization, skin rash, itching, skin hyperemia, alopecia.
Others: anemia.
Overdose
Overdose
None of the known few cases of overdose (maximum dose taken 450 mg) have shown specific symptoms.
Treatment: induce vomiting, take activated charcoal. Symptomatic therapy. Liver and renal functions and serum CPK levels should be monitored.
In case of myopathy with rhabdomyolysis and acute renal failure (a rare but severe side effect) the drug should be stopped immediately and the patient should be given a diuretic and sodium bicarbonate (intravenous infusion). Hemodialysis is indicated if necessary.
Rhabdomyolysis may cause hyperkalemia, which may be managed by intravenous calcium chloride or calcium gluconate administration, glucose and insulin infusion, use of potassium ion exchangers or, in severe cases, with hemodialysis.
Pregnancy use
Pregnancy use
Simvastatin may have adverse effects on the fetus and is contraindicated in pregnant women. There have been several reports of abnormalities in newborns whose mothers have taken Simvastatin.
Women of reproductive age taking Simvastatin should avoid conception. The use of Simvastatin is not recommended in women of childbearing age who do not use contraceptives. If pregnancy does occur during treatment, Simvastatin should be discontinued and the woman should be warned of the possible danger to the fetus.
There are no data on excretion of Simvastatin with maternal milk. If it is necessary to prescribe Simvastatin during breastfeeding, it should be taken into account that many drugs are excreted with breast milk, and there is a risk of severe reactions, so breastfeeding while taking the drug is not recommended.
Similarities
Similarities
Additional information
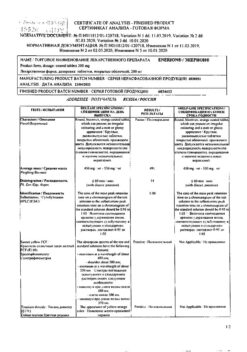
| Shelf life | 2 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Zentiva k.s., Czech Republic |
| Medication form | pills |
| Brand | Zentiva k.s. |
Related products
Buy Simvastatin Zentiva, tablets 40 mg, 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.