No products in the cart.
Signicef, eye drops 0.5% 5 ml
€5.88 €5.14
Description
The main active ingredient of the drug is levofloxacin, which is the L-isomer of the antibacterial agent of the II generation of fluoroquinolones group of Ofloxacin.
The drug is active against Gram-negative (Branhamella catarrhalis, Neisseria gonorrhoeae, Haemophilus influenzae, Pseudomonas aeruginosa) and Gram-positive (Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes) anaerobes. Another microflora sensitive to levofloxacin is Chlamydia trachomatis. The maximum concentration of levofloxacin achieved with the use of eye drops exceeds the values of the minimum inhibitory concentration of this active substance for sensitive microorganisms by more than a hundred times.
After instillation directly into the eye, levofloxacin is able to retain well in the tear film, causing persistent therapeutic action.
Pharmacokinetics
Levofloxacin is well maintained in the tear film after instillation into the eye. The concentration of levofloxacin in the tear fluid after a single dose (1 drop) quickly reaches high values and is maintained above the MIC for most sensitive ocular pathogens (less than or equal to 2 µg/ml) for at least 6 hours.
In studies on healthy volunteers, 5 of 6 subjects were shown to have levofloxacin concentrations of 2 µg/mL or higher 4 h after instillation. In 4 of 6 subjects this concentration was maintained 6 hours after instillation.
The mean plasma concentration of levofloxacin 1 h after administration ranged from 0.86 ng/ml in the first day to 2.05 ng/ml. Cmax of levofloxacin in plasma equal to 2.25 ng/ml was detected on the fourth day after two days of using the drug every 2 hours up to 8 times a day.
The Cmax of levofloxacin achieved on day 15 is more than 1,000 times lower than concentrations observed after oral administration of standard doses of levofloxacin.
Indications
Indications
infections of the ocular adnexa and anterior segment of the eye caused by flora sensitive to levofloxacin (treatment);
complications after surgical and laser ophthalmic operations (prevention).
Pharmacological effect
Pharmacological effect
The main active ingredient of the drug, levofloxacin, is the L-isomer of the second generation antibacterial agent of the fluoroquinolone group, ofloxacin.
The drug is active against gram-negative (Branhamella catarrhalis, Neisseria gonorrhoeae, Haemophilus influenzae, Pseudomonas aeruginosa) and gram-positive (Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes) anaerobes. Another microflora sensitive to levofloxacin is Chlamydia trachomatis. The maximum concentration of levofloxacin achieved when using eye drops exceeds the minimum inhibitory concentration of this active substance for sensitive microorganisms by more than a hundred times.
After instillation directly into the eye, levofloxacin is able to be well preserved in the tear film, causing a lasting therapeutic effect.
Pharmacokinetics
After instillation into the eye, levofloxacin is well preserved in the tear film. The concentration of levofloxacin in tear fluid after a single dose (1 drop) quickly reaches high values and is maintained above the MIC for most susceptible ocular pathogens (less than or equal to 2 μg/ml) for at least 6 hours.
In studies on healthy volunteers, it was shown that in 5 out of 6 subjects, levofloxacin concentrations were 2 μg/ml or higher 4 hours after instillation. In 4 out of 6 subjects this concentration remained 6 hours after instillation.
The average concentration of levofloxacin in blood plasma 1 hour after use is from 0.86 ng/ml on the first day to 2.05 ng/ml. Cmax of levofloxacin in plasma equal to 2.25 ng/ml was detected on the fourth day after two days of using the drug every 2 hours up to 8 times a day.
The Cmax of levofloxacin, achieved on day 15, was more than 1000 times lower than the concentrations observed after oral administration of standard doses of levofloxacin.
Special instructions
Special instructions
Signicef is not installed subconjunctivally or in the anterior chamber of the eyes.
When simultaneously using other ophthalmic agents with this drug, an interval between instillations of at least 15 minutes should be observed. Signicef should not be prescribed simultaneously with antacid drugs; the interval between the use of these drugs should be at least 2 hours.
When wearing soft hydrophilic lenses, Signicef is not recommended due to the presence of benzalkonium chloride in the preparation. This preservative can be absorbed by contact lenses, causing adverse effects on the eyes and causing discoloration of the lenses themselves.
To avoid contamination of the tip of the dropper or solution, during installations you should refrain from touching the eye.
Active ingredient
Active ingredient
Levofloxacin
Composition
Composition
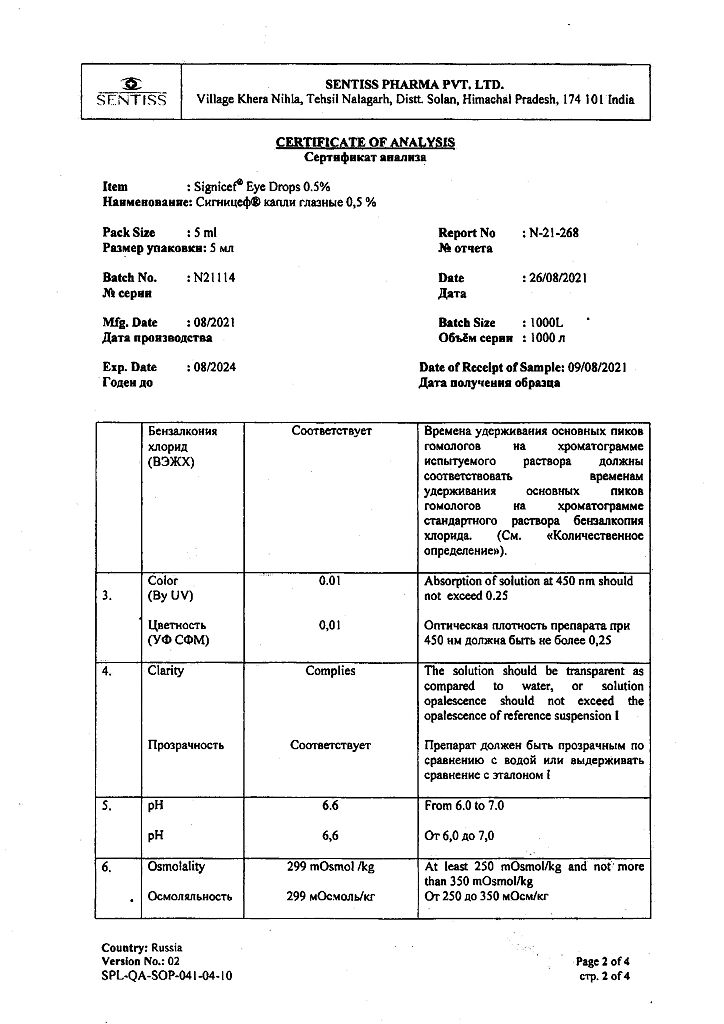
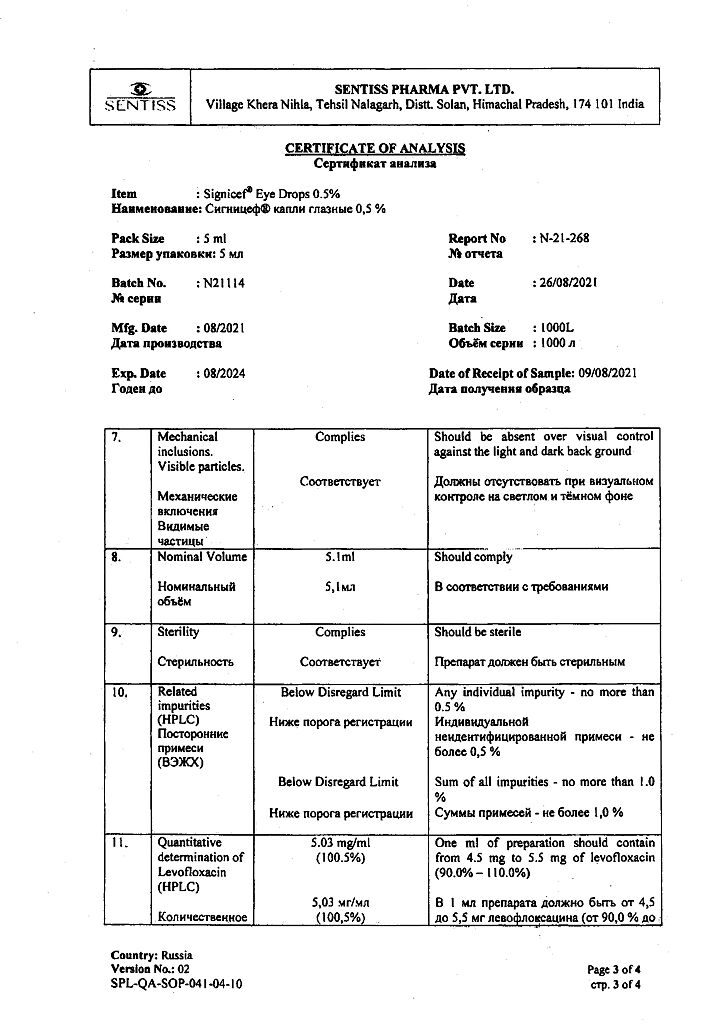
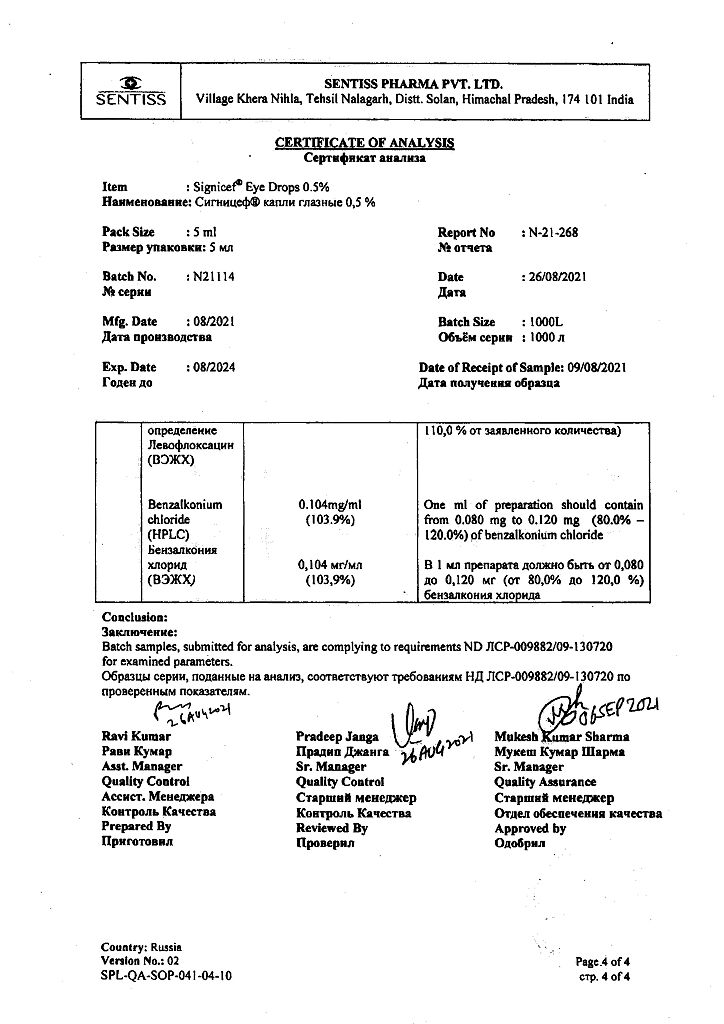
1 ml – levofloxacin (in the form of hemihydrate) 5 mg.
Excipients:
benzalkonium chloride,
hypromellose,
sodium chloride,
sodium hydroxide,
hydrochloric acid,
water d/i.
Pregnancy
Pregnancy
Contraindicated during pregnancy and breastfeeding, children under 1 year.
The drug should be prescribed with caution to children and adolescents under the age of 18 years.
Contraindications
Contraindications
pregnancy;
breast-feeding;
infants of the first year of life;
individual intolerance to quinolones or any of the components of the drug.
For children and adolescents under 18 years of age, the drug is prescribed with caution.
Side Effects
Side Effects
Side effects may occur in approximately 10% of patients. Frequent side effects (1-10% of patients) are decreased visual acuity and the appearance of mucous strands.
Rare side effects (0.1-1% of patients) – blepharitis, chemosis, papillary growths on the conjunctiva, swelling of the eyelids, discomfort in the eye, burning and itching in the eye, blurred vision, eye pain, conjunctival hyperemia, mucous discharge, conjunctival folliculosis, dry eye syndrome, eyelid erythema, contact dermatitis, photophobia and allergic reactions, headache, rhinitis.
Interaction
Interaction
No special studies have been conducted on the interaction of Signicef 0.5% eye drops.
Since the maximum plasma concentration of levofloxacin after topical application to the eye is at least 1000 times lower than after standard oral doses, interactions with other drugs characteristic of systemic use are clinically insignificant.
Overdose
Overdose
The total amount of levofloxacin contained in one vial of eye drops is too small to cause toxic reactions, even after accidental ingestion.
Symptoms: possible increased side effects.
Treatment: after topical application of an excess dose of Signicef eye drops 0.5%, the eyes should be rinsed with clean water at room temperature.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 30 °C (do not freeze)
Shelf life
Shelf life
2 years
Manufacturer
Manufacturer
Sentiss Pharma Pvt.Ltd, India
Additional information
| Shelf life | 2 years |
|---|---|
| Conditions of storage | In the dark place at a temperature not exceeding 30 °C (do not freeze) |
| Manufacturer | Sentiss Pharma Pvt.Ltd, India |
| Medication form | eye drops |
| Brand | Sentiss Pharma Pvt.Ltd |
Related products
Buy Signicef, eye drops 0.5% 5 ml with delivery to USA, UK, Europe and over 120 other countries.