No products in the cart.
Seroquel Prolong, 300 mg 60 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacodynamics
Quetiapine is an atypical antipsychotic drug. Quetiapine and its active metabolite N-dezalkylquetiapine interact with brain neutrotransmitter receptors. Quetiapine and N-dezalkylquetiapine exhibit high affinity for serotonin receptor types 5NT2 and dopamine receptor types D1 and D2 of the brain.
Higher selectivity for 5NT2 type serotonin receptors than for D2 type dopamine receptors, accounts for the major clinical antipsychotic properties of Seroquel Prolong and the low incidence of extrapyramidal side effects. In addition, N-dezalkylquetiapine exhibits a high affinity for the norepinephrine transporter. Quetiapine and N-dezalkylquetiapine have high affinity for histamine and α1 adrenoreceptors and less affinity for α2 adrenoreceptors and serotonin type 5NT1 receptors. Quetiapine shows no appreciable affinity for cholinergic muscarinic and benzodiazepine receptors.
Quetiapine shows antipsychotic activity in standard tests.
The specific contribution of the metabolite N-dezalkylquetiapine to the pharmacological activity of quetiapine has not been established.
The results of a study of extrapyramidal symptoms (EPS) in animals revealed that quetiapine causes mild catalepsy at doses that effectively block type D2 receptors. Quetiapine causes a selective decrease in the activity of mesolimbic A10 dopaminergic neurons compared to A9 nigrostriatal neurons involved in motor function.
Seroquel Prolong is well tolerated when taken at the recommended doses, including elderly patients.
In patients aged 66 to 89 years with dementia, taking Seroquel Prolong at doses of 50 to 300 mg/day decreases symptoms of depression.
The incidence of EPS and body weight in stable patients with schizophrenia does not increase with long-term therapy with Seroquel Prolong.
The DSM-IV (Diagnostic and Statistical Manual of Mental Disorders (4th ed.)) studies of major depressive disorder did not observe an increased risk of suicidal behavior and suicidal ideation when taking Seroquel Prolong compared to placebo.
Pharmacokinetics
Quetiapine is well absorbed from the GI tract. Cmax of quetiapine and N-dezalkylquetiapine in plasma is reached approximately 6 h after administration of Seroquel Prolong. The equilibrium molar concentration of the active metabolite N-dezalkylquivetiapine is 35% of that of quetiapine.
The pharmacokinetics of quetiapine and N-desalkylquetiapine are linear and dose-dependent when taking Seroquel Prolong at doses up to 800 mg once daily.
When taking Seroquel Prolong once daily at a dose equivalent to the daily dose of Seroquel taken in 2 doses, similar AUCs were observed, but Cmax was 13% lower. The AUC of the metabolite N-dezalkylquetiapine was 18% lower.
Studies of the effect of food intake on the bioavailability of quetiapine showed that eating a high-fat meal resulted in statistically significant increases in Cmax and AUC for Seroquel Prolong of approximately 50 and 20%, respectively. Low-fat dietary intake had no significant effect on the Cmax and AUC of quetiapine. It is recommended that Seroquel Prolong be taken once daily separately from meals.
About 83% of quetiapine is bound to plasma proteins.
CYP3A4 has been found to be a key isoenzyme in the cytochrome P450-mediated metabolism of vetiapine. N-dezalkylquetiapine is formed with the participation of CYP3A4 isoenzyme.
Quetiapine and some of its metabolites (including N-dezalkylquetiapine) have weak inhibitory activity against cytochrome P450 isoenzymes 1A2, 2C9, 2C19, 2D6 and 3A4, but only at concentrations 5-50 times higher than those observed at commonly used effective doses of 300-800 mg/day.
Based on in vitro results, concomitant use of quetiapine with other drugs should not be expected to result in clinically significant inhibition of cytochrome P450-mediated metabolism of other drugs.
The T1/2 of vetiapine and N-dezalkyl vetiapine is approximately 7 and 12 h, respectively. Approximately 73% of quetiapine is excreted in the urine and 21% in the feces. Quetiapine is actively metabolized in the liver, less than 5% of quetiapine is not metabolized and is excreted unchanged by the kidneys or in the feces.
There are no differences in pharmacokinetic parameters in men and women.
The average Cl of quetiapine in elderly patients is 30-50% less than in patients aged 18 to 65 years.
The mean plasma Cl of quetiapine is reduced by approximately 25% in patients with severe renal impairment (Cl creatinine2), but individual clearance rates are within the range of values found in healthy volunteers.
In patients with hepatic impairment (compensated alcoholic cirrhosis), the average plasma Cl of quetiapine is reduced by approximately 25%. Since quetiapine is extensively metabolized in the liver, patients with hepatic impairment may have increased plasma concentrations of quetiapine, which requires dose adjustments.
Indications
Indications
Schizophrenia, including relapse prevention in stable patients; bipolar disorders, including:
moderate and severe manic episodes in the structure of bipolar disorder;
severe episodes of depression in the structure of bipolar disorder;
prevention of relapses of bipolar disorders in patients with previous effective treatment with quetiapine for manic or depressive episodes in the structure of bipolar disorder.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Quetiapine is an atypical antipsychotic drug. Quetiapine and its active metabolite N-desalkylquetiapine interact with neurotransmitter receptors in the brain. Quetiapine and N-desalkylquetiapine exhibit high affinity for serotonin receptors type 5HT2 and dopamine receptors types D1 and D2 of the brain.
Higher selectivity for serotonin receptors of the 5HT2 type than for dopamine receptors of the D2 type determines the main clinical antipsychotic properties of the drug Seroquel Prolong and the low incidence of extrapyramidal side effects. In addition, N-desalkylquetiapine exhibits high affinity for the norepinephrine transporter. Quetiapine and N-desalkylquetiapine have high affinity for histamine and α1-adrenergic receptors and lower affinity for α2-adrenergic receptors and serotonin receptors of the 5HT1 type. Quetiapine does not show significant affinity for cholinergic muscarinic and benzodiazepine receptors.
In standard tests, quetiapine exhibits antipsychotic activity.
The specific contribution of the N-desalkylquetiapine metabolite to the pharmacological activity of quetiapine has not been established.
The results of a study of extrapyramidal symptoms (EPS) in animals revealed that quetiapine causes mild catalepsy in doses that effectively block D2 receptors. Quetiapine causes a selective decrease in the activity of mesolimbic A10 dopaminergic neurons compared to A9 nigrostriatal neurons involved in motor function.
Seroquel Prolong is well tolerated when taken in recommended doses, incl. elderly patients.
In patients aged 66 to 89 years with dementia, taking Seroquel Prolong in doses of 50 to 300 mg/day reduces symptoms of depression.
The frequency of EPS and body weight in stable patients with schizophrenia do not increase with long-term therapy with Seroquel Prolong.
In studies of major depressive disorder according to DSM-IV criteria (Diagnostic and Statistical Manual of Mental Disorders (4th ed.) – Handbook of Diagnostics and Statistics of Mental Disorders, 4th edition), no increased risk of suicidal behavior and suicidal ideation was observed when taking Seroquel Prolong compared to placebo.
Pharmacokinetics
Quetiapine is well absorbed from the gastrointestinal tract. Cmax of quetiapine and N-desalkylquetiapine in blood plasma is achieved approximately 6 hours after taking Seroquel Prolong. The equilibrium molar concentration of the active metabolite N-desalkylquetiapine is 35% of that of quetiapine.
The pharmacokinetics of quetiapine and N-desalkylquetiapine are linear and dose-dependent when taking Seroquel Prolong in doses of up to 800 mg once a day.
When taking Seroquel Prolong once daily at a dose equivalent to the daily dose of Seroquel taken in 2 doses, similar AUCs were observed, but Cmax was 13% less. The AUC value of the N-desalkylquetiapine metabolite was 18% less.
Studies of the effect of food intake on the bioavailability of quetiapine have shown that a high-fat meal leads to a statistically significant increase in Cmax and AUC for Seroquel Prolong by approximately 50 and 20%, respectively. A low-fat meal did not have a significant effect on the Cmax and AUC of quetiapine. It is recommended to take Seroquel Prolong 1 time per day separately from food.
Approximately 83% of quetiapine is bound to plasma proteins.
It has been established that CYP3A4 is a key isoenzyme in the metabolism of quetiapine, mediated by cytochrome P450. N-desalkylquetiapine is formed with the participation of the CYP3A4 isoenzyme.
Quetiapine and some of its metabolites (including N-desalkylquetiapine) have weak inhibitory activity against cytochrome P450 isoenzymes 1A2, 2C9, 2C19, 2D6 and 3A4, but only at concentrations 5–50 times higher than those observed at commonly used effective doses of 300–800 mg/day.
Based on in vitro results, concomitant use of quetiapine with other drugs should not be expected to result in clinically significant inhibition of cytochrome P450-mediated metabolism of other drugs.
T1/2 of quetiapine and N-desalkylquetiapine is about 7 and 12 hours, respectively. Approximately 73% of quetiapine is excreted in urine and 21% in feces. Quetiapine is extensively metabolized in the liver; less than 5% of quetiapine is not metabolized and is excreted unchanged by the kidneys or feces.
There are no differences in pharmacokinetic parameters between men and women.
The average Cl of quetiapine in elderly patients is 30–50% less than in patients aged 18 to 65 years.
The mean plasma Cl of quetiapine is reduced by approximately 25% in patients with severe renal impairment (Cl creatinine2), but individual clearance rates are within the range of values found in healthy volunteers.
In patients with liver failure (compensated alcoholic cirrhosis), the average plasma Cl of quetiapine is reduced by approximately 25%. Since quetiapine is extensively metabolized in the liver, in patients with liver failure, plasma concentrations of quetiapine may increase, which requires dose adjustment.
Special instructions
Special instructions
Drowsiness
During therapy with Seroquel Prolong, drowsiness and associated symptoms, such as sedation, may occur. In clinical studies involving patients with depression as part of bipolar disorder, somnolence typically developed during the first three days of therapy. The severity of this side effect was generally minor or moderate. If severe sleepiness develops, patients with depression as part of bipolar disorder may require more frequent visits to the doctor for 2 weeks after the onset of sleepiness or until symptoms improve. In some cases, it may be necessary to discontinue therapy with Seroquel Prolong.
Patients with cardiovascular diseases
Caution should be exercised when prescribing Seroquel Prolong to patients with cardiovascular and cerebrovascular diseases and other conditions predisposing to hypotension. During therapy with Seroquel Prolong, orthostatic hypotension may occur, especially during dose titration at the beginning of therapy. If orthostatic hypotension occurs, dose reduction or slower titration may be necessary.
Dysphagia and aspiration were observed during therapy with Seroquel Prolong. The cause-and-effect relationship between the occurrence of aspiration pneumonia and the use of Seroquel Prolong has not been established. However, caution should be exercised when prescribing the drug to patients at risk of aspiration pneumonia.
Seizures
There were no differences in the incidence of seizures in patients taking quetiapine or placebo. However, as with the treatment of other antipsychotic drugs, caution is recommended when treating patients with a history of seizures.
Extrapyramidal symptoms
There was an increase in the incidence of EPS in adult patients with depression in the structure of bipolar disorder when taking quetiapine for depressive episodes compared to placebo. However, when treating patients with schizophrenia and mania in the structure of bipolar disorder with quetiapine, there was no increase in the incidence of EPS compared to placebo.
Tardive dyskinesia
If symptoms of tardive dyskinesia develop, it is recommended to reduce the dose of the drug or gradually discontinue it. Symptoms of tardive dyskinesia may worsen or even occur after you stop taking the drug.
Neuroleptic malignant syndrome
While taking antipsychotic drugs, incl. quetiapine, neuroleptic malignant syndrome may develop. Clinical manifestations of the syndrome include hyperthermia, altered mental status, muscle rigidity, lability of the autonomic nervous system, and increased creatine phosphokinase activity. In such cases, it is necessary to stop taking Seroquel Prolong and carry out appropriate treatment.
Severe neutropenia
In clinical studies of quetiapine, cases of severe neutropenia (neutrophil count 9/L) were infrequently observed. Most cases of severe neutropenia occurred several months after initiation of quetiapine therapy. No dose-dependent effect was found. Leukopenia and/or neutropenia resolved after discontinuation of quetiapine therapy. Possible risk factors for the occurrence of neutropenia are a previous low white blood cell count and a history of drug-induced neutropenia. In patients with a neutrophil count of 9/L, quetiapine should be discontinued. The patient should be observed for possible symptoms of infection and the neutrophil count should be monitored (until the level exceeds 1.5 109/L).
Interaction with other drugs
The use of Seroquel Prolong in combination with powerful inducers of the liver enzyme system, such as carbamazepine and phenytoin, helps to reduce the concentration of quetiapine in plasma and may reduce the effectiveness of therapy with Seroquel Prolong.
Prescribing Seroquel Prolong to patients receiving inducers of the liver enzyme system is possible only if the expected benefit from therapy with Seroquel Prolong outweighs the risk associated with discontinuation of the drug – an inducer of liver enzymes. Changing the dose of drugs that are inducers of microsomal enzymes should be gradual. If necessary, they can be replaced with drugs that do not induce microsomal enzymes (for example, valproic acid drugs).
Hyperglycemia
While taking quetiapine, hyperglycemia or exacerbation of diabetes mellitus may develop in patients with a history of diabetes mellitus. Clinical monitoring of patients with diabetes mellitus and patients with risk factors for developing diabetes mellitus is recommended.
Lipid concentration
While taking quetiapine, it is possible to increase the concentration of triglycerides and cholesterol and decrease the concentration of HDL.
QT prolongation
There was no relationship between taking quetiapine and a persistent increase in the absolute value of the QT interval. However, prolongation of the QT interval has been observed with drug overdose. Caution should be exercised when prescribing quetiapine, as with other antipsychotic drugs, to patients with cardiovascular disease and a history of QT prolongation. It is also necessary to be careful when prescribing quetiapine simultaneously with drugs that prolong the QTc interval, other antipsychotics, especially in the elderly, patients with congenital long QT syndrome, chronic heart failure, myocardial hypertrophy, hypokalemia or hypomagnesemia.
Acute reactions associated with drug withdrawal
If quetiapine is abruptly discontinued, the following acute reactions (withdrawal syndrome) may occur: nausea, vomiting, insomnia, headache, dizziness and irritability. Therefore, it is recommended to discontinue the drug gradually, over at least one or two weeks.
Elderly patients with dementia
Seroquel Prolong is not indicated for the treatment of psychoses associated with dementia.
Some atypical antipsychotics in randomized placebo-controlled trials increased the risk of cerebrovascular events in patients with dementia by approximately 3-fold. The mechanism for this increased risk has not been studied. A similar risk of increased incidence of cerebrovascular complications cannot be excluded for other antipsychotic drugs or other patient groups. Seroquel Prolong should be used with caution in patients at risk of stroke.
An analysis of the use of atypical antipsychotics for the treatment of psychosis associated with dementia in elderly patients revealed an increase in mortality in the group of patients receiving drugs of this group compared with the placebo group. Two 10-week placebo-controlled studies of quetiapine in a similar group of patients (n=710; mean age: 83 years; age range: 56-99 years) showed that mortality in the quetiapine group was 5.5%, in the placebo group – 3.2%. The causes of death observed in these patients were consistent with those expected for this population. No causal relationship has been identified between quetiapine treatment and the risk of increased mortality in elderly patients with dementia.
Suicide/suicidal ideation or clinical worsening
Depression is associated with an increased risk of suicidal ideation, self-harm and suicide (suicide-related events). This risk persists until significant remission occurs. Because it may take several weeks or more for the patient’s condition to improve from the start of treatment, patients should be under close medical supervision until improvement occurs. According to generally accepted clinical experience, the risk of suicide may increase in the early stages of remission.
Other mental disorders for which quetiapine is prescribed are also associated with an increased risk of suicide-related events. In addition, such conditions may be comorbid with a depressive episode. Thus, the precautions used when treating patients with a depressive episode should also be taken when treating patients with other mental disorders.
Patients with a history of suicidal events, as well as patients who clearly express suicidal thoughts before starting therapy, are at increased risk of suicidal intent and suicide attempts and should be carefully monitored during treatment.
In short-term, placebo-controlled studies, across all indications and in all age groups, the incidence of suicide events was 0.9% for both quetiapine (61/6270) and placebo (27/3047).
In these studies in patients with schizophrenia, the risk of suicide-related events was 1.4% (3/212) for quetiapine and 1.6% (1/62) for placebo in patients aged 18–24 years; 0.8% (13/1663) for quetiapine and 1.1% (5/463) for patients over 25 years of age; 1.4% (2/147) for quetiapine and 1.3% (1/75) for placebo in patients under 18 years of age.
In patients with manic bipolar disorder, the risk of suicide-related events was 0% (0/67) for quetiapine and 0% (1/57) for placebo in patients aged 18–24 years; 1.2% (6/496) for quetiapine and 1.2% (6/503) for placebo in patients over 25 years of age; 1% (2/193) for quetiapine and 0% (0/90) for placebo in patients under 18 years of age (see Precautions).
In patients with depressed bipolar disorder, the risk of suicide-related events was 3% (7/233) for quetiapine and 0% (0/120) for placebo in patients aged 18–24 years; 1.8% (19/1616) for quetiapine and 1.8% (11/622) for placebo in patients over 25 years of age. No studies have been conducted in patients with depression due to bipolar disorder under the age of 18 years.
Seroquel Prolong may cause drowsiness, therefore, during the treatment period, patients are not recommended to work with dangerous mechanisms, incl. Driving is not recommended.
Active ingredient
Active ingredient
Quetiapine
Composition
Composition
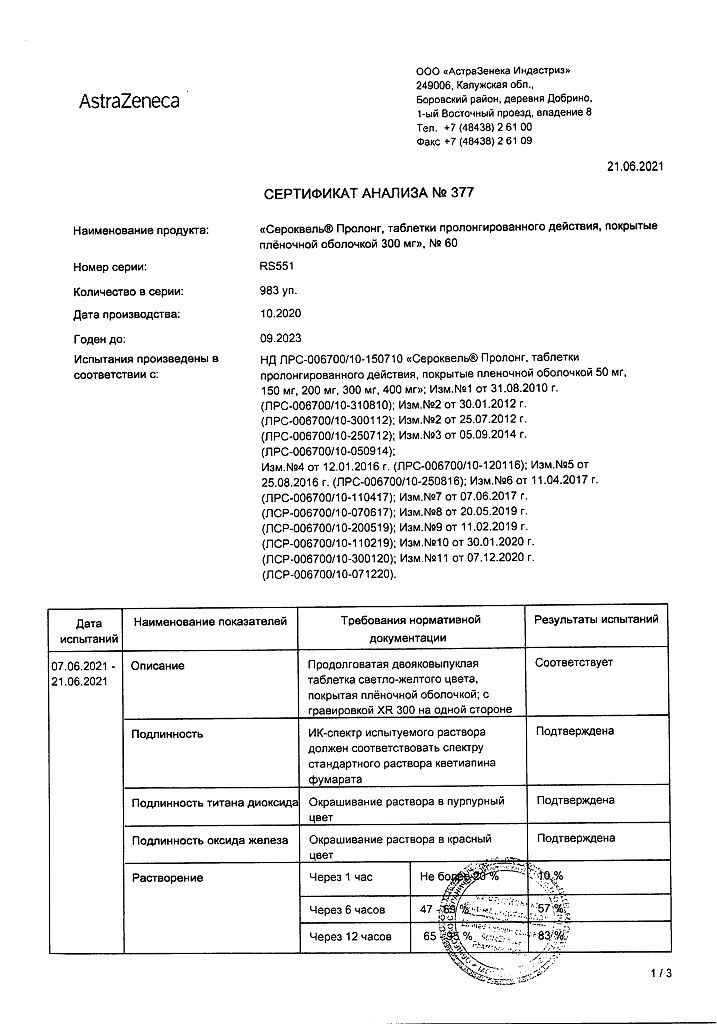
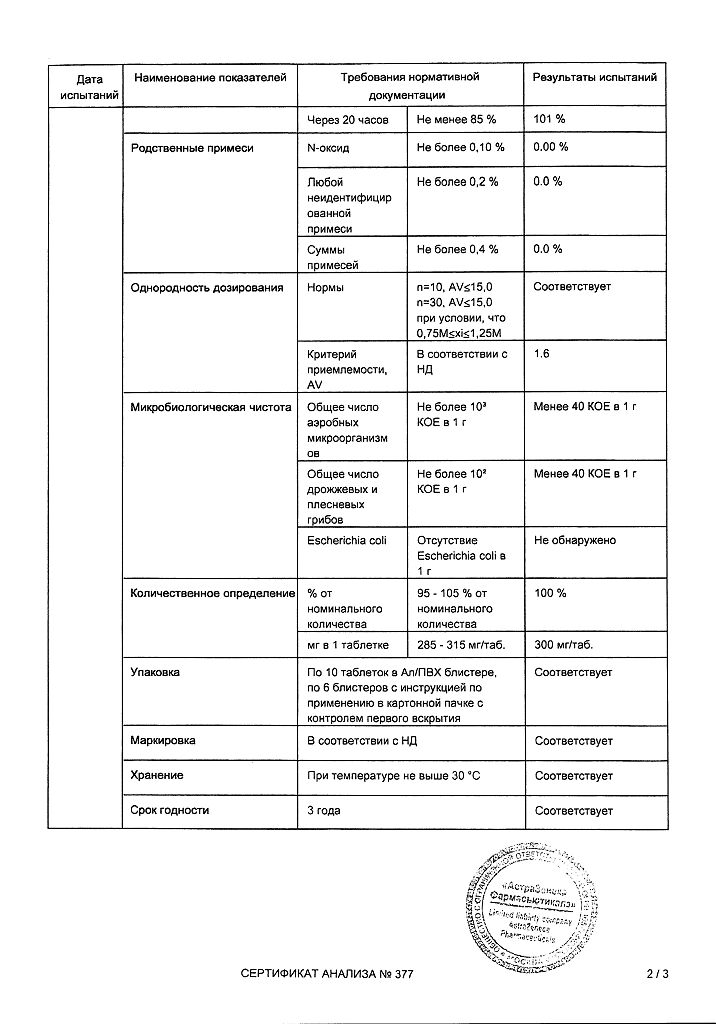
1 extended-release film-coated tablet contains:
active substance:
quetiapine (as fumarate) 300 mg,
excipients:
lactose monohydrate;
MCC;
sodium citrate dihydrate;
hypromellose;
magnesium stearate,
shell composition:
hypromellose; macrogol 400; titanium dioxide E171; iron oxide yellow dye E172
Pregnancy
Pregnancy
The safety and effectiveness of quetiapine in pregnant women have not been established. As a result, Seroquel Prolong can be used during pregnancy only if the expected benefit to the woman justifies the potential risk to the fetus.
The extent of quetiapine excretion in human milk is unknown. Women should avoid breastfeeding while taking Seroquel Prolong.
Contraindications
Contraindications
hypersensitivity to the drug Seroquel prolong.
lactase deficiency, glucose-galactose malabsorption and galactose intolerance;
combined use with cytochrome P450 inhibitors, such as azole antifungals, erythromycin, clarithromycin and nefazodone, as well as protease inhibitors;
age under 18 years (despite the fact that the effectiveness and safety of Seroquel Prolong in children and adolescents aged 10–17 years have been studied in clinical studies).
With caution:
cardiovascular and cerebrovascular diseases or other conditions predisposing to arterial hypotension;
old age;
liver failure;
history of seizures.
Side Effects
Side Effects
The most common side effects of Seroquel Prolong are drowsiness, dizziness, dry mouth, mild asthenia, constipation, tachycardia, orthostatic hypotension and dyspepsia.
Taking Seroquel Prolong, like other antipsychotic drugs, may be accompanied by weight gain, fainting, the development of neuroleptic malignant syndrome, leukopenia, neutropenia and peripheral edema.
The frequency of adverse reactions is given in the following gradation: very often – ≥1/10; often – ≥1/100,
Very often
From the side of the central nervous system
dizziness1, 4, 17, drowsiness 2, 17, headache
From the gastrointestinal tract
dry mouth
General disorders
withdrawal syndrome1, 10
Changes in laboratory and instrumental parameters
increased concentration of triglycerides11, total cholesterol (mainly LDL)12, decreased concentration of HDL cholesterol18, increased body weight9, decreased hemoglobin concentration
Often
From the hematopoietic system
leukopenia1
From the side of the central nervous system
dysarthria, unusual and nightmare dreams, fainting1, 4, 17, extrapyramidal symptoms1, 13, increased appetite
From the cardiovascular system
tachycardia1, 4, orthostatic hypotension1, 4
From the side of the organ of vision
blurred vision
From the respiratory system
rhinitis
From the gastrointestinal tract
constipation, dyspepsia
General disorders
mild asthenia, irritability, peripheral edema
Changes in laboratory and instrumental parameters
increased activity of liver transaminases (AST, ALT) 3, decreased number of neutrophils, hyperglycemia7, increased concentration of prolactin in blood serum15
Uncommon
From the blood system
eosinophilia
From the immune system
hypersensitivity reactions
From the side of the central nervous system
seizures1, restless legs syndrome, tardive dyskinesia
From the gastrointestinal tract
dysphagia8
Changes in laboratory and instrumental parameters
increased creatine phosphokinase activity not associated with neuroleptic malignant syndrome; increased GGT3 activity, thrombocytopenia14
Rarely
From the gastrointestinal tract
jaundice6
From the reproductive system
priapism, galactorrhea16
Common disorders:
neuroleptic malignant syndrome1
Changes in laboratory and instrumental parameters
increased activity of creatine phosphokinase15
Very rarely
From the immune system
anaphylactic reactions6
Metabolic disorders
diabetes mellitus1, 5, 6
From the side of the central nervous system
tardive dyskinesia6
From the gastrointestinal tract
hepatitis6
From the skin and subcutaneous tissues
angioedema6, Stevens-Johnson syndrome6
Unspecified frequency
From the hematopoietic system
neutropenia1
See Special Instructions.
Drowsiness usually occurs within the first 2 weeks after initiation of therapy and, as a rule, resolves with continued use of Seroquel Prolong.
An asymptomatic increase in the activity of AST, ALT and GGT in the blood serum is possible, usually reversible with continued use of the drug Seroquel Prolong.
Like other antipsychotic drugs and α1-blockers, Seroquel Prolong often causes orthostatic hypotension, which is accompanied by dizziness, tachycardia, and in some cases fainting, especially at the beginning of therapy.
Very rare cases of decompensation of diabetes mellitus have been reported.
The frequency of this side effect was assessed based on the results of post-marketing surveillance of the use of the drug Seroquel Prolong.
An increase in fasting blood glucose ≥126 mg/dL (≥7 mmol/L) or postprandial blood glucose ≥200 mg/dL (≥11.1 mmol/L), at least once.
A higher incidence of dysphagia with quetiapine compared with placebo was observed only in patients with depression as part of bipolar disorder.
More than 7% excess of initial body weight. Mostly occurs at the beginning of therapy.
When studying the withdrawal syndrome in short-term placebo-controlled clinical trials of the drug Seroquel Prolong in monotherapy, the following symptoms were noted: insomnia, nausea, headache, diarrhea, vomiting, dizziness and irritability. The incidence of withdrawal symptoms decreased significantly 1 week after discontinuation of the drug.
Increased triglyceride concentrations ≥200 mg/dL (≥2.258 mmol/L) in patients ≥18 years of age or ≥150 mg/dL (≥1.694 mmol/L) in patients
Increased total cholesterol concentrations ≥240 mg/dL (≥6.2064 mmol/L) in patients ≥18 years of age or ≥200 mg/dL (≥5.172 mmol/L) in patients
See further text of the Instructions.
Decrease in platelet count ≤100·109/l, at least with a single determination.
No association with neuroleptic malignant syndrome. According to clinical studies.
Increased prolactin concentration in patients ≥18 years of age: >20 mcg/L (≥869.56 pmol/L) in men; >30 mcg/L (≥1304.34 pmol/L) in women.
May cause a fall.
Reducing HDL cholesterol concentrations
QT prolongation, ventricular arrhythmia, sudden death, cardiac arrest, and torsade de pointes (TdP) are considered adverse effects of antipsychotic drugs.
The incidence of EPS in short-term clinical trials in schizophrenia and mania in bipolar disorder was comparable in the quetiapine and placebo groups (patients with schizophrenia: 7.8% in the quetiapine group and 8% in the placebo group; mania in bipolar disorder: 11.2% in the quetiapine group and 11.4% in the placebo group).
The frequency of EPS in short-term clinical trials for depression in the structure of bipolar disorder in the quetiapine group was 8.9%, in the placebo group – 3.8%. At the same time, the frequency of individual symptoms of EPS (such as akathisia, extrapyramidal disorders, tremor, dyskinesia, dystonia, anxiety, involuntary muscle contractions, psychomotor agitation and muscle rigidity) was usually low and did not exceed 4% in each of the therapeutic groups. In long-term clinical studies of quetiapine in schizophrenia and bipolar disorder, the incidence of EPS was comparable in the quetiapine and placebo groups.
During quetiapine therapy, a slight dose-dependent decrease in the concentration of thyroid hormones, in particular total thyroxine (T4) and free T4, may be observed. The maximum decrease in total and free T4 was recorded on the 2nd and 4th weeks of therapy with Seroquel Prolong, without a further decrease in hormone concentrations during long-term treatment. In almost all cases, the concentration of total and free T4 returned to the initial level after discontinuation of therapy with Seroquel Prolong, regardless of the duration of treatment. A slight decrease in total triiodothyronine (T3) and reverse T3 was observed only when using high doses. The concentration of thyroxine-binding globulin (TBG) remained unchanged, and no increase in TSH concentration was observed.
Interaction
Interaction
Caution should be exercised when using Seroquel Prolong in combination with other drugs that affect the central nervous system, as well as with alcohol.
Cytochrome P450 isoenzyme (CYP) 3A4 is the main isoenzyme responsible for the metabolism of quetiapine through the cytochrome P450 system. When studied in healthy volunteers, coadministration of quetiapine (25 mg) with ketoconazole, a CYP3A4 inhibitor, resulted in a 5- to 8-fold increase in quetiapine AUC.
Therefore, the combined use of quetiapine and inhibitors of cytochrome CYP3A4 is contraindicated. During quetiapine therapy, it is not recommended to drink grapefruit juice.
In a pharmacokinetic study, the use of quetiapine at various dosages before or simultaneously with carbamazepine led to a significant increase in quetiapine Cl and, accordingly, a decrease in AUC, by an average of 13%, compared to taking quetiapine without carbamazepine. In some patients, the decrease in AUC was even more pronounced. This interaction was accompanied by a decrease in plasma concentrations of quetiapine and could reduce the effectiveness of therapy with Seroquel® Prolong. The combined use of quetiapine with phenytoin, another inducer of the liver microsomal system, was accompanied by an even more pronounced (approximately 450%) increase in quetiapine clearance. The use of Seroquel® Prolong by patients receiving inducers of the liver enzyme system is possible only if the expected benefit from therapy with Seroquel® Prolong outweighs the risk associated with discontinuation of the drug – an inducer of liver enzymes. Changing the dose of drugs that induce microsomal enzymes should be gradual. If necessary, they can be replaced with drugs that do not induce microsomal enzymes (for example, valproic acid drugs).
The pharmacokinetics of quetiapine were not significantly altered by concomitant use of the antidepressant imipramine (CYP2D6 inhibitor) or fluoxetine (CYP3A4 and CYP2D6 inhibitor).
The pharmacokinetics of quetiapine does not change significantly when used simultaneously with antipsychotic drugs – risperidone or haloperidol. However, simultaneous administration of quetiapine and thioridazine resulted in an increase in quetiapine Cl by approximately 70%.
The pharmacokinetics of quetiapine does not change significantly with simultaneous use of cimetidine.
With a single dose of 2 mg of lorazepam while taking quetiapine at a dose of 250 mg 2 times a day, the clearance of lorazepam is reduced by approximately 20%.
The pharmacokinetics of lithium preparations does not change with simultaneous use of quetiapine. There were no clinically significant changes in the pharmacokinetics of valproic acid and quetiapine with the combined use of semisodium valproate and quetiapine.
Pharmacokinetic studies examining the interaction of Seroquel Prolong with drugs used for cardiovascular diseases have not been conducted.
Caution should be exercised when using the drug Seroquel Prolong in combination with drugs that can cause electrolyte imbalance and prolongation of the QTc interval.
Quetiapine did not cause induction of hepatic enzyme systems involved in the metabolism of phenazone.
False-positive results of screening tests for methadone and tricyclic antidepressants using enzyme-linked immunosorbent assays have been reported in patients taking quetiapine. To confirm the screening results, a chromatographic study is recommended.
Overdose
Overdose
Symptoms. A death was reported with 13.6 g of quetiapine in a patient participating in a clinical trial, and a death was reported with 6 g of quetiapine in a post-marketing study. At the same time, a case of taking quetiapine in a dose exceeding 30 g without death was described.
There are reports of extremely rare cases of quetiapine overdose, leading to an increase in the QTc interval, death or coma.
In patients with a history of severe cardiovascular disease, the risk of side effects from overdose may increase.
Symptoms noted during overdose were mainly due to an increase in the known pharmacological effects of the drug, such as drowsiness and sedation, tachycardia and decreased blood pressure.
Treatment. There are no specific antidotes to quetiapine. In cases of severe intoxication, one should be aware of the possibility of overdose of several drugs. It is recommended to take measures aimed at maintaining respiratory and cardiovascular function, ensuring adequate oxygenation and ventilation. Gastric lavage (after intubation if the patient is unconscious) and the use of activated charcoal and laxatives may help eliminate unabsorbed quetiapine, but the effectiveness of these measures has not been studied.
Close medical observation should continue until the patient’s condition improves.
Storage conditions
Storage conditions
At a temperature not exceeding 30 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
AstraZeneca Pharmaceuticals LP, USA
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | AstraZeneca Pharmaceuticals LP, USA |
| Medication form | slow-release tablets |
| Brand | AstraZeneca Pharmaceuticals LP |
Other forms…
Related products
Buy Seroquel Prolong, 300 mg 60 pcs with delivery to USA, UK, Europe and over 120 other countries.