No products in the cart.
Serlift, 50 mg 28 pcs
€8.84 €7.74
Out of stock
(E-mail when Stock is available)
Description
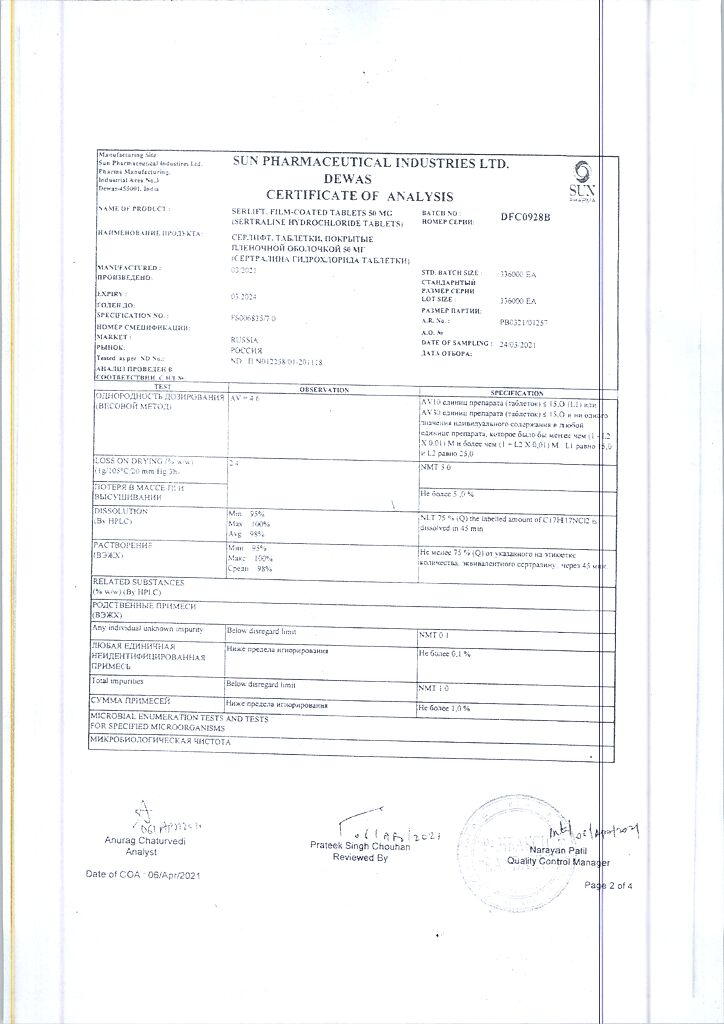
Serlift is an antidepressant derivative of naphthylamine. It is a selective blocker of serotonin neuronal reuptake in the brain.
The neuronal takeover of norepinephrine and dopamine is practically unaffected.
Serlift has no specific affinity for adreno- and m-cholinoreceptors, GABA receptors, dopamine, histamine, serotonin or benzodiazepine receptors. It does not inhibit MAOIs.
Causes anorexia and is effective in obsessive compulsive states.
Indications
Indications
Treatment of depressive states of various origins in patients with mono- and bipolar affective disorders. Prevention of relapse of episodes of depression.
Pharmacological effect
Pharmacological effect
Sirlift is an antidepressant, a naphthylamine derivative. Selective blocker of neuronal reuptake of serotonin in the brain.
The neuronal uptake of norepinephrine and dopamine is practically unaffected.
Surlift does not have a specific affinity for adrenergic and m-cholinergic receptors, GABA receptors, dopamine, histamine, serotonin or benzodiazepine receptors. Does not inhibit MAO.
Causes anorexia, effective for obsessive states.
Special instructions
Special instructions
Use with caution if there is a history of drug abuse or dependence, impaired liver function, impaired renal function, epileptic seizures, or weight loss.
Should not be used in patients undergoing electroconvulsive therapy. The use of sertraline is possible no earlier than 14 days after discontinuation of MAO inhibitors.
During the treatment period, avoid drinking alcohol.
Safety for use in pediatrics has not been established.
Impact on the ability to drive vehicles and operate machinery
During the treatment period, activities that require increased attention and high speed of psychomotor reactions should be avoided.
Active ingredient
Active ingredient
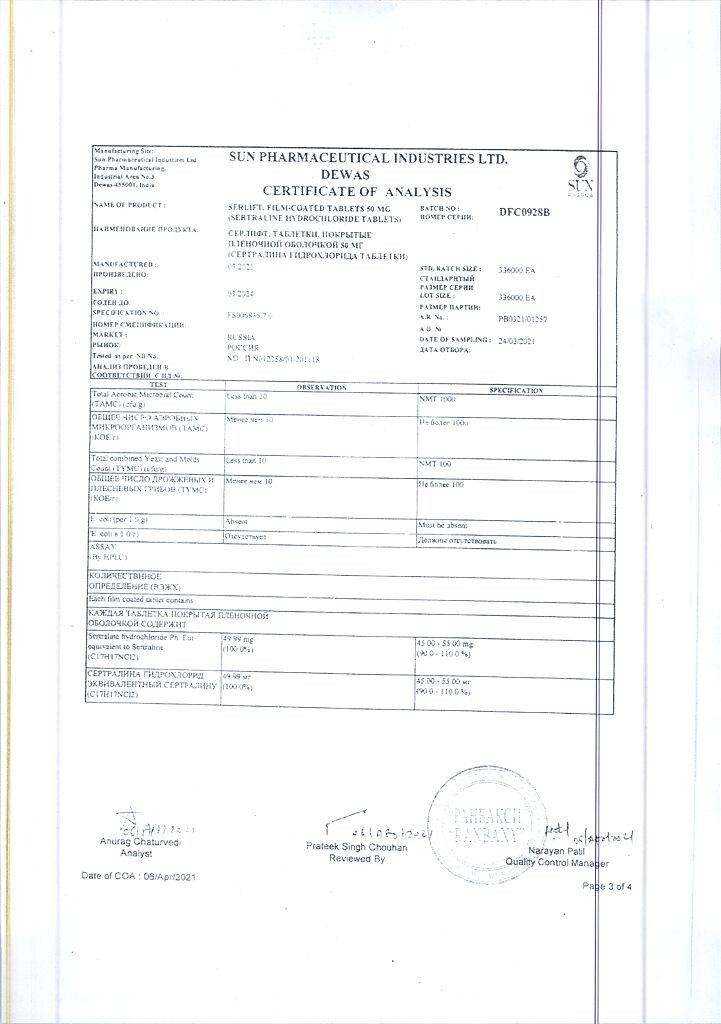
Sertraline
Composition
Composition
1 tab. contains sertraline 50 mg
Pregnancy
Pregnancy
Adequate and well-controlled studies of the safety of sertraline during pregnancy have not been conducted, so use is possible only in cases where the expected benefit to the mother outweighs the possible risk to the fetus.
It is unknown whether sertraline is excreted in breast milk, so use during lactation is not recommended. Some studies have shown that in infants whose mothers received sertraline while breastfeeding, its plasma levels are negligible or undetectable, while concentrations in breast milk exceed those in the mother’s blood.
Women of childbearing age should use reliable methods of contraception during treatment with sertraline.
Experimental studies have not revealed the teratogenic or mutagenic effects of sertraline. However, at doses approximately 2.5 to 10 times the maximum daily clinical dose, sertraline caused a delay in ossification of fetal bone tissue, possibly as a result of effects on the mother. When sertraline was administered at doses approximately 5 times the maximum clinical dose, a decrease in neonatal survival was observed.
Contraindications
Contraindications
Concomitant use with MAO inhibitors, increased sensitivity to sertraline.
Side Effects
Side Effects
From the side of the central nervous system: dizziness, drowsiness, headache, insomnia, feeling of fatigue, weakness, tremor; rarely – manic or hypomanic state, anxiety, restlessness, visual impairment.
From the cardiovascular system: rarely – redness of the skin with a feeling of heat or warmth, a feeling of heartbeat.
From the digestive system: loss of appetite, diarrhea, dry mouth, nausea, cramps in the stomach or intestines, flatulence; rarely – constipation, vomiting.
Metabolism: increased sweating.
From the reproductive system: rarely – decreased potency.
Allergic reactions: rarely – fever, skin rash, urticaria or itching.
Interaction
Interaction
With simultaneous use of coumarin derivatives with anticoagulants, the prothrombin time significantly increases.
With simultaneous use, sertraline can displace other drugs from binding to plasma proteins, as a result of which the plasma concentration of the corresponding active substance increases and the risk of side effects increases.
When used simultaneously with drugs whose metabolism occurs with the participation of the CYP2D6 isoenzyme, it is possible to increase the plasma concentration of these drugs due to inhibition of the CYP2D6 isoenzyme under the influence of sertraline.
With the simultaneous use of MAO inhibitors (including selegiline, moclobemide), the development of serotonin syndrome (hyperthermia, muscle rigidity, myoclonus, as well as manifestations of instability of the mental and physiological state of the body, up to the development of delirium and coma) is possible.
When used simultaneously with lithium salts, tremor may increase. When used simultaneously with desipramine, it is possible to increase the concentration of desipramine in the blood plasma; with cimetidine – a significant decrease in the clearance of sertraline.
Storage conditions
Storage conditions
In a dry place, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Sun Pharmaceutical Industries Ltd, India
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry place, at a temperature not exceeding 25 °C |
| Manufacturer | Sun Pharmaceutical Industries Ltd, India |
| Medication form | pills |
| Brand | Sun Pharmaceutical Industries Ltd |
Other forms…
Related products
Buy Serlift, 50 mg 28 pcs with delivery to USA, UK, Europe and over 120 other countries.