No products in the cart.
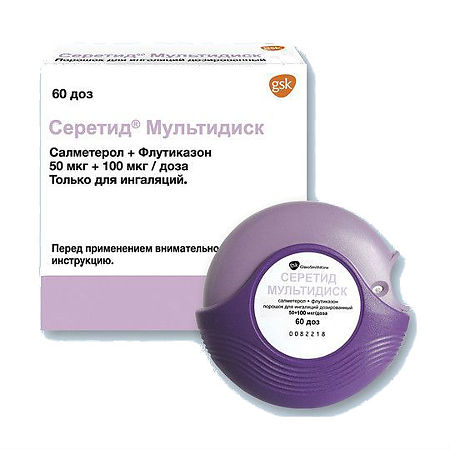
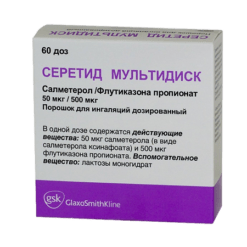
Seretide Multidisc, 50 mcg+100 mcg/dose 60 doses
€33.41 €27.84
Description
The drug Seretid Multidisc has anti-asthmatic, bronchodilator, anti-inflammatory action.
Pharmacodynamics
The drug Seretide Multidisc is a combined preparation that contains salmeterol and fluticasone propionate, which have different mechanisms of action. Salmeterol prevents bronchospasm, fluticasone propionate improves pulmonary function and prevents exacerbations. The drugs may be an alternative for patients who simultaneously receive a β2 adrenoreceptor agonist and an inhaled GCS.
Salmeterol is a selective long-acting (up to 12 hours) β2-adrenoreceptor agonist that has a long side chain that binds to the outer domain of the receptor.
The pharmacological properties of salmeterol provide protection against histamine-induced bronchoconstriction and longer bronchodilation (lasting at least 12 hours) than short-acting β2-adrenoreceptor agonists. The onset of bronchodilator effect is within 10-20 minutes. Salmeterol is a strong and long-acting inhibitor of mast cell mediators such as histamine, LT and PG D2 release from human lung tissue.
Salmeterol inhibits the early and late phases of response to inhaled allergens; the latter lasts for more than 30 h after administration of 1 dose, a time when the bronchodilator effect is no longer present. A single dose of salmeterol attenuates bronchial hyperresponsiveness. This indicates that salmeterol, in addition to its bronchodilator activity, has an additional action, the clinical significance of which has not been definitively established. This mechanism of action is different from the anti-inflammatory effect of GCS. In therapeutic doses, salmeterol has no effect on CCC.
Fluticasone propionate belongs to the group of GCS for local use and when administered by inhalation in the recommended doses it has a pronounced anti-inflammatory and anti-allergic effect in the lungs, which leads to reduction of clinical symptoms and decreases the frequency of exacerbations of diseases accompanied by airway obstruction. It restores the patient’s response to bronchodilators, allowing to reduce the frequency of their use. The action of fluticasone propionate is not accompanied by the adverse reactions typical for systemic GCS.
Long-term use of inhaled fluticasone propionate at maximum recommended doses keeps daily adrenal hormone secretion within normal limits in both adults and children. After switching patients receiving other inhaled GCSs to fluticasone propionate, daily secretion of adrenal cortical hormones gradually improves despite prior and current intermittent use of oral steroids. This indicates recovery of adrenal function against the background of inhaled use of fluticasone propionate. With prolonged use of fluticasone propionate, adrenal cortical reserve function also remains within normal limits, as evidenced by a normal increase in cortisol production in response to appropriate stimulation (it should be considered that a residual decrease in adrenal reserve caused by prior therapy may persist for a long time).
A study of 318 adult patients with persistent bronchial asthma showed that when using a doubled dose of Seretide and Seretide Multidisc for 14 days (regardless of the dose of components in the drug) there was a slight increase in the frequency of adverse events associated with the action of β-adrenomimetic (tremor – 1 patient (1%), 0 patients – at the usual dose; palpitations – 6 patients (6%), 1 patient (
Pharmacokinetics
. When co-inhaled, salmeterol and fluticasone propionate do not affect the pharmacokinetics of each other, so the pharmacokinetic characteristics of each component of Seretide Multidisc can be considered separately.
Even though the plasma concentrations of salmeterol and fluticasone propionate are very low, interactions with other substrates and CYPZA4 isoenzyme inhibitors cannot be excluded.
Salmeterol: acts locally in pulmonary tissue, so its plasma content does not correlate with the therapeutic effect. Data on its pharmacokinetics are very limited due to technical problems: when inhaled at therapeutic doses, its Cmax in plasma is extremely low (about 200 pg/mL and below). After repeated inhalations of salmeterol xinaphoate, hydroxynaphthoic acid, Css of which is about 10 pg/ml, can be detected in the blood. These concentrations are 1000 times lower than the equilibrium levels observed in toxicity studies.
Fluticasone propionate: The absolute bioavailability of inhaled fluticasone propionate in healthy subjects varies depending on the inhaler used (when using salmeterol/fluticasone propionate with a metered dose inhaler aerosol, it is 5.3% of the nominal dose). In patients with bronchial asthma and COPD, lower plasma concentrations of fluticasone propionate are observed. Systemic absorption occurs predominantly through the lungs, and it is faster at first, but then slows down.
A portion of the inhaled dose may be swallowed, but this portion contributes minimally to systemic absorption due to the drug’s low water solubility and due to its presystemic metabolism. Bioavailability from the gastrointestinal tract is less than 1%. As the inhaled dose increases, there is a linear increase in plasma concentrations of fluticasone propionate. The distribution of fluticasone propionate is characterized by rapid plasma clearance (1150 mL/min), a large Vss (approximately 300 L), and a final T1/2 of approximately 8 h. Fluticasone propionate has a relatively high degree of binding to plasma proteins (91%). It is rapidly eliminated from the blood, mainly as a result of metabolism by CYP3A4 isoenzyme to inactive carboxyl metabolite.
The renal clearance of unchanged fluticasone propionate is negligible (
Extracted through the gastrointestinal tract, mainly as a hydroxylated metabolite.
Indications
Indications
Treatment of bronchial asthma in patients who are indicated for combination therapy with a long-acting β2-agonist and an inhaled corticosteroid:
in patients with insufficient disease control on the background of constant monotherapy with inhaled corticosteroids with periodic use of a short-acting β2-adrenergic agonist;
in patients with adequate disease control during therapy with an inhaled corticosteroid and a long-acting β2-adrenergic agonist;
as initial maintenance therapy in patients with persistent bronchial asthma (daily occurrence of symptoms, daily use of drugs for rapid relief of symptoms) if there are indications for prescribing GCS to achieve disease control;
Maintenance therapy for COPD in patients with a forced inspiratory volume (FEV1) value
Pharmacological effect
Pharmacological effect
Seretide Multidisk has anti-asthmatic, bronchodilator, anti-inflammatory effects.
Pharmacodynamics
The drug Seretide Multidisk is a combination drug containing salmeterol and fluticasone propionate, which have different mechanisms of action. Salmeterol prevents the occurrence of bronchospasm, fluticasone propionate improves pulmonary function and prevents exacerbations. The drugs may be an alternative for patients who are simultaneously receiving a β2-adrenergic agonist and inhaled corticosteroids.
Salmeterol is a selective, long-acting (up to 12 hours) β2-adrenergic receptor agonist that has a long side chain that binds to the outer domain of the receptor.
The pharmacological properties of salmeterol provide protection against histamine-induced bronchoconstriction and longer-lasting bronchodilation (lasting at least 12 hours) than short-acting β2-adrenergic receptor agonists. The onset of the bronchodilator effect is within 10–20 minutes. Salmeterol is a strong and long-acting inhibitor of the release of mast cell mediators such as histamine, LT and PG D2 from human lung tissue.
Salmeterol inhibits the early and late phases of the response to inhaled allergens; the latter lasts more than 30 hours after administration of 1 dose, i.e. at a time when the bronchodilator effect is no longer present. A single administration of salmeterol weakens the hyperreactivity of the bronchial tree. This indicates that salmeterol, in addition to its bronchodilator activity, has an additional effect, the clinical significance of which has not been fully established. This mechanism of action differs from the anti-inflammatory effect of GCS. At therapeutic doses, salmeterol has no effect on CCC.
Fluticasone propionate belongs to the group of corticosteroids for topical use and, when administered in inhalation in recommended doses, has a pronounced anti-inflammatory and antiallergic effect in the lungs, which leads to a decrease in clinical symptoms and a decrease in the frequency of exacerbations of diseases accompanied by airway obstruction. Restores the patient’s response to bronchodilators, allowing to reduce the frequency of their use. The effect of fluticasone propionate is not accompanied by adverse reactions characteristic of systemic corticosteroids.
With long-term use of inhaled fluticasone propionate in the maximum recommended doses, the daily secretion of adrenal hormones remains within normal limits in both adults and children. After switching patients receiving other inhaled corticosteroids to fluticasone propionate, the daily secretion of adrenal hormones gradually improves, despite previous and current intermittent use of oral steroids. This indicates restoration of adrenal function with inhaled use of fluticasone propionate. With long-term use of fluticasone propionate, the reserve function of the adrenal cortex also remains within normal limits, as evidenced by the normal increase in cortisol production in response to appropriate stimulation (it must be taken into account that the residual decrease in adrenal reserve caused by previous therapy may persist for a long time).
A study conducted among 318 adult patients with persistent bronchial asthma showed that when using a double dose of Seretide and Seretide Multidisk for 14 days (regardless of the dose of the components in the drug), there was a slight increase in the frequency of adverse events associated with the action of β-adrenergic agonists (tremor – 1 patient (1%), 0 patients – with the usual dose; rapid heartbeat – 6 patients (6%), 1 patient (
Pharmacokinetics
When administered together by inhalation, salmeterol and fluticasone propionate do not affect each other’s pharmacokinetics, therefore the pharmacokinetic characteristics of each component of Seretide Multidisk can be considered separately.
Even despite the very low plasma concentrations of salmeterol and fluticasone propionate, interactions with other substrates and inhibitors of the CYP3A4 isoenzyme cannot be excluded.
Salmeterol: acts locally in the lung tissue, so its plasma levels do not correlate with the therapeutic effect. Data on its pharmacokinetics are very limited due to technical problems: when inhaled in therapeutic doses, its Cmax in plasma is extremely low (about 200 pg/ml and below). After repeated inhalations of salmeterol xinafoate, hydroxynaphthoic acid can be detected in the blood, the Css of which is about 10 pg/ml. These concentrations are 1000 times lower than equilibrium levels observed in toxicity studies.
Fluticasone propionate: The absolute bioavailability of inhaled fluticasone propionate in healthy subjects varies depending on the inhaler used (when using salmeterol/fluticasone propionate using a metered dose inhalation aerosol, it is 5.3% of the nominal dose). In patients with bronchial asthma and COPD, lower plasma concentrations of fluticasone propionate are observed. Systemic absorption occurs primarily through the lungs, and is initially faster but then slows down.
Part of the inhalation dose may be swallowed, but this part makes a minimal contribution to systemic absorption due to the low solubility of the drug in water and due to its first-pass metabolism. Bioavailability from the gastrointestinal tract is less than 1%. As the inhalation dose increases, a linear increase in the plasma concentration of fluticasone propionate is observed. The distribution of fluticasone propionate is characterized by rapid plasma clearance (1150 ml/min), a large Vss (about 300 l) and a final half-life of approximately 8 hours. Fluticasone propionate has a relatively high degree of plasma protein binding (91%). It is rapidly eliminated from the blood, mainly as a result of metabolism under the action of the CYP3A4 isoenzyme to an inactive carboxyl metabolite.
The renal clearance of unchanged fluticasone propionate is negligible (
It is excreted through the gastrointestinal tract, mainly in the form of a hydroxylated metabolite.
Special instructions
Special instructions
Seretide Multidisk is intended for long-term treatment of the disease, and not for the relief of attacks. To relieve attacks, patients should be prescribed short-acting inhaled bronchodilators (for example, salbutamol), which patients are advised to always have with them.
If paradoxical bronchospasm develops, you should immediately use a short-acting inhaled bronchodilator, discontinue Seretide® Multidisk and begin, if indicated, alternative therapy. Treatment of bronchial asthma is recommended to be carried out in stages, monitoring the patient’s clinical response to treatment and lung function. The patient must be taught how to use the inhaler correctly. The severity and frequency of deepening of the voice and candidiasis can be reduced by rinsing the mouth with water after inhalation of Seretide Multidisc. For candidiasis, antifungal drugs are prescribed for topical use, while therapy with Seretide Multidisk is continued.
In elderly people and patients with renal failure or impaired liver function, no dose reduction is required. The drug can be used for initial maintenance therapy in patients with persistent bronchial asthma (daily occurrence of symptoms or daily use of drugs to relieve attacks) if there are indications for the use of corticosteroids and their approximate dosage has been determined. More frequent use of short-acting bronchodilators to relieve symptoms indicates worsening disease control, and in such situations the patient should consult a doctor. Sudden and progressive deterioration in control of bronchospastic syndrome is potentially life-threatening. In such situations, medical supervision is necessary. If the dose of Seretide Multidisk used does not provide adequate control of the disease, then additional administration of GCS may be required, and if an exacerbation is caused by an infection, then antibiotics are prescribed. Due to the risk of exacerbation, sudden discontinuation of Seretide Multidisk should be avoided; the dose of the drug should be reduced gradually under the supervision of a physician. When using any inhaled GCS, it is possible to develop systemic effects (suppression of adrenal function, growth retardation in children and adolescents, decreased bone mineral density, cataracts and glaucoma), especially with long-term use in high doses, but the likelihood of such effects occurring is much lower than with treatment with oral forms of GCS. Given this, the dose of inhaled corticosteroids should be titrated to the minimum that ensures the maintenance of effective control. In emergency and planned stressful situations, it is always necessary to remember the possibility of suppression of adrenal function and the need to use GCS.
When carrying out resuscitation measures or surgical interventions, it is necessary to determine the degree of adrenal insufficiency. Some patients may experience individual high sensitivity to inhaled corticosteroids. Due to possible adrenal insufficiency, special caution should be exercised and regular monitoring of adrenal function indicators when transferring patients who have taken oral corticosteroids to treatment with inhaled fluticasone propionate. When transferring patients from taking systemic corticosteroids to inhalation therapy, allergic reactions (for example, allergic rhinitis, eczema), which were previously suppressed by systemic corticosteroids, may occur. In such situations, it is recommended to carry out symptomatic treatment with antihistamines and/or topical drugs (including corticosteroids for topical use). Cancellation of systemic corticosteroids against the background of inhaled fluticasone propionate should be carried out gradually. Patients should carry a card with them indicating that they may need additional corticosteroids in various stressful situations.
Use in pediatrics: it is recommended to monitor the growth dynamics of children who receive long-term therapy with inhaled corticosteroids. There are currently no data on the use of Seretide Multidisc in children under 4 years of age.
Monitoring laboratory parameters: in patients with exacerbation of bronchial asthma, hypoxia, it is necessary to monitor the concentration of potassium in the blood plasma. There are very rare reports of increased blood glucose levels; this should be kept in mind when prescribing a combination of salmeterol with fluticasone propionate to patients with diabetes mellitus.
Active ingredient
Active ingredient
Salmeterol, Fluticasone
Composition
Composition
Active ingredients:
salmeterol (in the form of xinafoate) 50 mcg,
fluticasone propionate 100 mcg;
Excipients:
lactose monohydrate.
Pregnancy
Pregnancy
During pregnancy and lactation (breastfeeding), Seretide can be prescribed only if the expected benefit to the mother outweighs any possible risk to the fetus or child.
Contraindications
Contraindications
Hypersensitivity to the components of the drug Seretide Multidisc; children under 4 years of age.
With caution: pulmonary tuberculosis, fungal, viral or bacterial infections of the respiratory system, thyrotoxicosis, pheochromocytoma, diabetes mellitus, uncontrolled hypokalemia, idiopathic hypertrophic subaortic stenosis, uncontrolled arterial hypertension, arrhythmias, prolongation of the QT interval on the ECG, ischemic heart disease, hypoxia of various origins, cataracts, glaucoma, hypothyroidism, osteoporosis, pregnancy, lactation.
Interaction
Interaction
Due to the risk of developing bronchospasm, the use of selective and non-selective beta-blockers should be avoided unless it is really necessary and justified.
In diseases accompanied by reversible airway obstruction, the use of both non-selective and cardioselective beta-blockers should be avoided, unless it is really necessary and justified. When fluticasone propionate is used in the form of inhalations, its concentration in the blood plasma is low due to intensive metabolism during the “first pass” through the liver under the influence of the CYP3A4 isoenzyme and high systemic clearance.
This makes a clinically significant interaction involving fluticasone propionate unlikely. Caution must be exercised when using known CYP3A4 inhibitors and fluticasone propionate simultaneously, since in such situations the plasma levels of the latter may increase. Ritonavir (a highly active inhibitor of the CYP3A4 isoenzyme) can cause a significant increase in the concentration of fluticasone propionate in the blood plasma, as a result of which serum cortisol concentrations are significantly reduced.
There are reports of clinically significant drug interactions in patients who simultaneously received fluticasone propionate and ritonavir, which was manifested by the development of Cushing’s syndrome and suppression of adrenal function. Given this, the simultaneous use of fluticasone propionate and ritonavir should be avoided, unless the potential benefit of combination therapy for the patient outweighs the risk of developing systemic side effects of GCS.
Other inhibitors of the CYP3A4 isoenzyme cause a negligible (erythromycin) and insignificant (ketoconazole) increase in plasma fluticasone propionate levels, with virtually no decrease in serum cortisol concentrations. Despite this, caution is recommended when using fluticasone propionate concomitantly with strong CYP3A4 inhibitors (eg, ketoconazole), since such combinations may increase the plasma concentrations of fluticasone propionate.
When used simultaneously with the drug Seretide® Multidisk, xanthine derivatives, corticosteroids and diuretics increase the risk of developing hypokalemia (especially in patients with exacerbation of bronchial asthma, during hypoxia); MAO inhibitors and tricyclic antidepressants increase the risk of side effects from the cardiovascular system. Seretide Multidisk is compatible with cromoglycic acid.
Overdose
Overdose
Symptoms: tremor, headache and tachycardia caused by the action of salmeterol; temporary inhibition of the hypothalamic-pituitary-adrenal system, which is caused by the action of fluticasone. With prolonged inhalation of the drug Seretide Multidisk in excessively high doses, noticeable suppression of adrenal function is possible.
There are rare reports of acute adrenal crisis, which occurs predominantly in children receiving Seretide Multidisk in excessively high doses for a long time (several months or years). Acute adrenal crisis is characterized by hypoglycemia, accompanied by confusion and/or convulsions. Situations that may trigger an acute adrenal crisis include trauma, surgery, infection, or a rapid reduction in the dose of fluticasone propionate contained in Seretide Multidisc.
Treatment: symptoms caused by the action of salmeterol should be relieved by administering an antidote – a cardioselective beta-blocker. In cases where it is necessary to discontinue Seretide Multidisk due to an overdose of salmeterol in its composition, the patient should be prescribed an appropriate replacement GCS.
Symptoms caused by the action of fluticasone propionate usually do not require emergency treatment, since in most cases normal adrenal function is restored within a few days. In case of chronic overdose, it is recommended to monitor the reserve function of the adrenal cortex. To avoid overdose, patients should not use Seretide Multidisc in doses higher than recommended. It is important to regularly assess the effectiveness of therapy and reduce the dose of Seretide Multidisk to the minimum level that ensures effective control of the symptoms of the disease.
Storage conditions
Storage conditions
At temperatures below 30 °C
Shelf life
Shelf life
18 months
Manufacturer
Manufacturer
Glaxo Wellcome Production, France
Additional information
| Shelf life | 18 months |
|---|---|
| Conditions of storage | At temperatures below 30 °C |
| Manufacturer | Glaxo Wellcome Production, France |
| Medication form | metered inhalation powder |
| Brand | Glaxo Wellcome Production |
Other forms…
Related products
Buy Seretide Multidisc, 50 mcg+100 mcg/dose 60 doses with delivery to USA, UK, Europe and over 120 other countries.