No products in the cart.
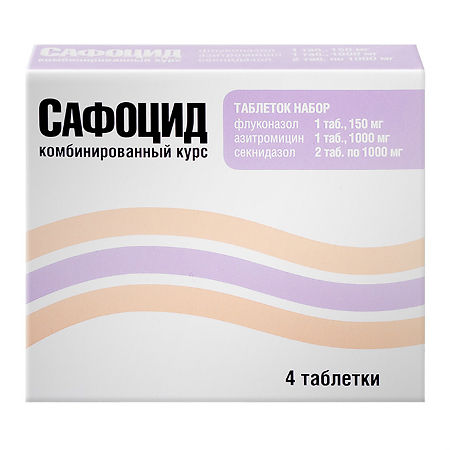
Safocid, set, tablets 4 pcs.
€1.00
Out of stock
(E-mail when Stock is available)
Description
Safocid is a combined kit containing an antifungal drug, an antibiotic and an antibacterial drug with antiprotozoal activity.
Fluconazole
Antifungal agent, has highly specific action, inhibiting the activity of cytochrome P450-dependent fungal enzymes.
Blocks conversion of lanosterol of fungal cells into ergosterol; increases cell membrane permeability, disrupts cell growth and replication.
Fluconazole, while being highly selective for cytochrome P450 of fungi, does not inhibit these enzymes in humans (compared to itraconazole, clotrimazole, econazole and ketoconazole it inhibits cytochrome P450 dependent oxidative processes in human liver microsomes to a lesser extent).
Does not have antiandrogenic activity.
It is active in opportunistic mycoses, including those caused by Candida spp. (including generalized candidiasis against immunosuppression), Cryptococcus neoformans and Coccidioides immitis
(including intracranial infections), Microsporum spp. and Trichophyton spp.; in endemic mycoses caused by Blastomyces dermatidis, Histoplasma capsulation (including immunosuppression).
Azithromycin
A broad-spectrum antibacterial agent, azalid, acts bacteriostatically. Binding to 50S subunit of ribosomes, it inhibits peptide translocase at the translation stage, inhibits protein synthesis, inhibits bacterial growth and multiplication, in high concentrations it has bactericidal effect.
It is active against extra- and intracellular pathogens.
Azithromycin-sensitive microorganisms:
Aerobic Gram-positive microorganisms – Staphylococcus aureus (methicillin-sensitive strains), Streptococcus pneumoniae (penicillin-sensitive strains), Streptococcus pyogenes;
aerobic gram-negative microorganisms – Haemophilus influenzae, Haemophilus parainfluenzae, Legionella pneumophila, Moraxella catarrhalis, Pasteurella multocida, Neisseria gonorrhoeae;
anaerobic microorganisms – Clostridium perfringens, Fusobacterium spp., Prevotella spp., Porphyromonas spp.;
other microorganisms – Chlamydia trachomatis, Chlamydia pneumoniae, Chlamydia psittaci, Mycoplasma pneumoniae, Mycoplasma hominis, Borrelia burgdorferi.
Microorganisms with acquired resistance to azithromycin:aerobic gram-positive microorganisms – Streptococcus pneumoniae (penicillin-resistant strains and strains with moderate sensitivity to penicillin).
Microorganisms with natural resistance: aerobic gram-positive microorganisms – Enterococcus faecalis, Staphylococcus aureus (methicillin-resistant strains), Staphylococcus epidermidis (methicillin-resistant strains); anaerobic microorganisms – Bacteroides fragilis.
Cases of cross-resistance between Streptococcus pneumoniae, Streptococcus pyogenes (group A beta-haemolytic streptococcus), Enterococcus faecalis and Staphylococcus aureus have been described.
including Staphylococcus aureus (methicillin-resistant strains) to erythromycin, azithromycin, other macrolides and lincosamides.
Secnidazole
The antimicrobial bactericidal agent is a synthetic derivative of nitroimidazole.
It is active against obligate anaerobic bacteria (spore- and non-spore-forming), causative agents of some protozoal infections: Trichomonas spp., Giardia lamblia, Entamoeba histolytica. It is not active against aerobic bacteria.
It interacts with the DNA of the microbial cell, causes disruption of the helical structure, strand breakage, suppression of nucleic acid synthesis and cell death. Causes sensitization to alcohol (tetura-like action).
Pharmacokinetics
Fluconazole
Intake and distribution
Absorption – high, bioavailability – 90%.
The absorption of the drug taken orally is not affected by concomitant eating.
After oral administration on an empty stomach of 150 mg Tmax is 0.5-1.5 h, Cmax of fluconazole in plasma is 90% of its concentration in plasma in case of intravenous administration in the same dose.
The binding to plasma proteins is 11-12%. Plasma concentrations are in direct relation to the dose.
Fluconazole penetrates well into all body fluids. The concentration of the active substance in breast milk, joint fluid, saliva, sputum and peritoneal fluid is similar to that in plasma.
It penetrates well into the cerebrospinal fluid; in fungal meningitis the concentration in the cerebrospinal fluid is about 85% of that in plasma.
In sweat fluid, epidermis and stratum corneum (selective accumulation), concentrations higher than serum concentrations are achieved. Vd approaches the total water content of the body.
Metabolism and excretion
The T1/2 of fluconazole is about 30 h. It is an inhibitor of CYP2C9 isoenzyme in the liver. It is excreted mainly by the kidneys (80% unchanged, 11% – as metabolites).
The clearance of fluconazole is proportional to creatinine clearance.
Pharmacokinetics in special clinical cases
The pharmacokinetics of fluconazole is significantly dependent on the functional state of the kidneys, with an inverse relationship between T1/2 and CK.
After hemodialysis for 3 h plasma concentration of fluconazole decreases by 50%.
Azithromycin
Intake and distribution
Asithromycin is rapidly absorbed from the gastrointestinal tract due to its stability in acidic environment and lipophilicity. Bioavailability after a single dose of 500 mg is 37% (effect of “first passage” through the liver).
After oral administration of 500 mg, Cmax is 0.4 mg/l, Tmax is 2.5-2.9 hours.
In tissues and cells, the concentration is 10-50 times higher than in blood serum. Vd – 31.1 l/kg.
It easily passes the histohematic barriers. It penetrates well into the respiratory tract, urogenital organs and tissues, prostate, skin and soft tissues; it accumulates in low pH environment, in lysosomes (which is especially important for eradication of intracellularly located pathogens).
It is also transported by phagocytes, polymorphonuclear leukocytes and macrophages (with no significant effect on their function).
It penetrates through cell membranes and generates high concentrations in cells. Concentrations in the foci of infection are significantly higher (by 24-34%) than in healthy tissues and correlate with the severity of inflammatory edema.
In the focus of inflammation it persists in effective concentrations for 5-7 days after the last dose. The binding to plasma proteins is 7-50% (inversely proportional to the concentration in blood).
Metabolism and excretion
It is demethylated in the liver, the resulting metabolites are inactive. Plasma clearance is 630 ml/min.
Pharmacokinetics in special clinical cases
Eating alters pharmacokinetics: Administration of azithromycin in tablet form increases Cmax by 31% with no change in AUC.
In elderly men (65-85 years old) pharmacokinetic parameters do not change, in women Cmax increases (by 30-50%).
Secnidazole
Intake and distribution
Absorption is high, bioavailability is 80%. After a single oral dose of 2 g, Tmax is 4 hours.
The drug penetrates through the placental barrier and is excreted with the breast milk.
Metabolism and excretion
Metabolized in the liver.
Extracted in the urine within 72 hours (16% of the dose).
Indications
Indications
Combined genitourinary tract sexually transmitted infections:
Composition
Composition
1 tablet contains:
The active ingredient:
Fluconazole150 mg;
Excipients:
MCC – 153 mg;
Calcium hydrophosphate – 14.5 mg;
Croscarmellose sodium – 5 mg;
Magnesium stearate – 4 mg;
Colloidal silicon dioxide – 1 mg,
Punsenium dye (Ponceau 4R) (E124) – 1.06 mg.
Azithromycin
1 tablet contains:
The active ingredient:
Azithromycin dihydrate1048 mg;
Excipients:
Sodium lauryl sulfate, 12 mg;
Croscarmellose sodium, 37.24 mg;
Povidone K30, 40 mg;
Magnesium stearate – 16 mg;
Colloidal silicon dioxide – 2 mg;
Filmed film sheath:
Hypromellose (hydroxypropyl methylcellulose) – 24.5 mg;
Diethyl phthalate – 3.8 mg;
Talc – 5.2 mg;
Titanium dioxide – 6 mg;
Macrogol 4000 – 4.2 mg;
Punsenic dye (Ponceau 4R) (E124) – 1.06 mg;
Secnidazole
1 tablet contains:
The active ingredient:
Secnidazole1000 mg;
Excipients:
Corn starch, 95 mg;
MCC, 130 mg;
Colloidal silicon dioxide, 6 mg;
Sodium carboxymethyl starch, 20 mg;
Povidone (PVPK30) – 2.5 mg;
Talk – 11.5 mg;
Magnesium stearate – 10 mg;
Film coating:
Hypromellose (hydroxypropyl methylcellulose) – 27.1 mg;
Diethyl phthalate – 4 mg;
Talc – 5.7 mg;
Titanium dioxide – 11.2 mg;
Macrogol 4000 – 4.6 mg.
How to take, the dosage
How to take, the dosage
Overly, once. Take all 4 tablets included in the blister at the same time, taking into account meals (since the absorption of azithromycin changes with meals, it is better to take it an hour before a meal or 2 hours after a meal).
Interaction
Interaction
Special Instructions
Special Instructions
Contraindications
Contraindications
Side effects
Side effects
Overdose
Overdose
Cases of overdose of Safocid tablets set have not been described. In case of overdose, consult a doctor immediately.
Fluconazole
Symptoms: hallucinations, paranoid behavior.
Treatment: symptomatic; gastric lavage (if necessary), forced diuresis. Hemodialysis within 3 hours reduces fluconazole concentration in blood plasma by approximately 50%.
Asithromycin
Symptoms: nausea, temporary hearing loss, vomiting, diarrhea.
Treatment: symptomatic; administration of activated charcoal, control of vital functions.
Secnidazole
In case of suspected overdose, supportive and symptomatic treatment should be given; gastric lavage, administration of activated charcoal.
Pregnancy use
Pregnancy use
Safocid is contraindicated in pregnancy and during lactation (breastfeeding).
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | Store in a place protected from light and moisture at a temperature not exceeding 30 ° C. Keep out of reach of children. |
| Manufacturer | Laika Labs Limited, India |
| Medication form | pills |
| Brand | Laika Labs Limited |
Related products
Buy Safocid, set, tablets 4 pcs. with delivery to USA, UK, Europe and over 120 other countries.