No products in the cart.
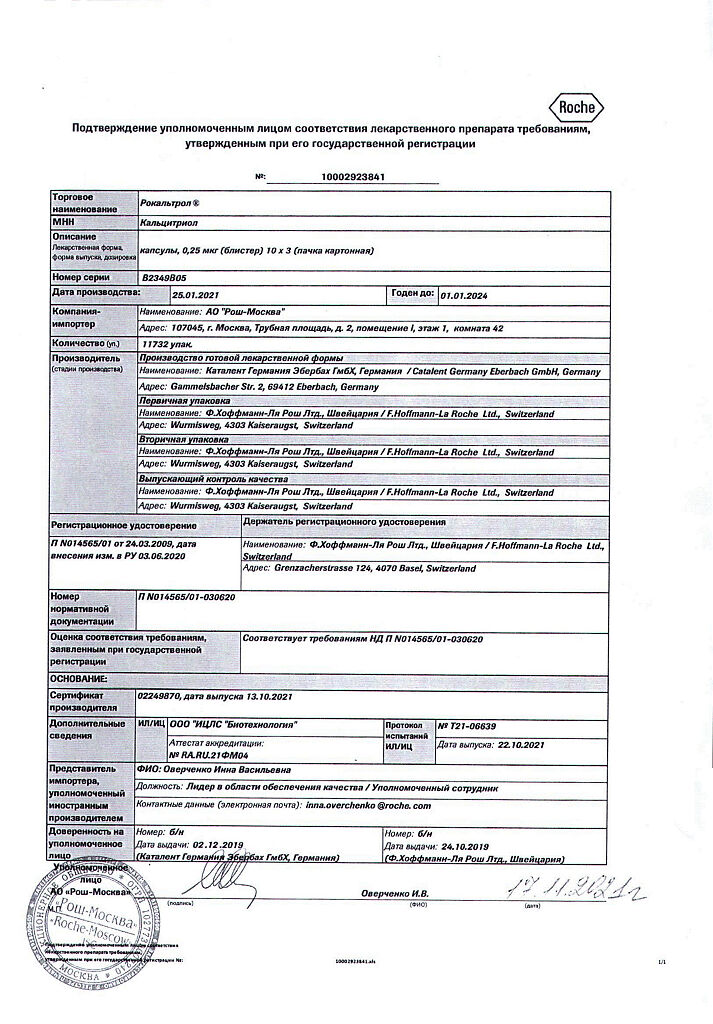
Rocaltrol, 0.25 mcg capsules 30 pcs
€8.88 €7.00
Out of stock
(E-mail when Stock is available)
Description
Rocaltrol – regulates calcium metabolism, stimulates osteogenesis, and normalizes calcium content in bone tissue.
Calcitriol is one of the most important active metabolites of vitamin D 3 . Calcitriol promotes the absorption of calcium in the intestine and regulates bone mineralization. The pharmacological effect of a single dose of calcitriol lasts 3-5 days.
The key role of calcitriol in regulation of calcium metabolism, consisting in stimulation of skeletal osteoblasts activity, is a reliable pharmacological basis for its therapeutic action in osteoporosis.
In individuals with severe renal insufficiency the synthesis of endogenous calcitriol is reduced or may even stop altogether. Calcitriol deficiency plays a major role in the occurrence of renal osteodystrophy.
In patients with renal osteodystrophy, Rocaltrol (synthetic calcitriol) normalizes reduced intestinal calcium absorption, eliminates hypocalcemia, elevated serum levels of alkaline phosphatase and parathormone. It reduces pain in bones and muscles, and eliminates the histological changes that occur in fibrous osteitis and other disorders of mineralization.
In patients with postoperative and idiopathic hypoparathyroidism and pseudohypoparathyroidism, Rocaltrol reduces hypocalcemia and its clinical manifestations.
In patients with vitamin-D-dependent rickets, the concentration of calcitriol in the serum is low or completely absent. Taking into account insufficient endogenous calcitriol synthesis in kidneys, taking Rocaltrol can be considered as replacement therapy in these patients.
In patients with vitamin-D-resistant rickets and hypophosphatemia with low plasma calcitriol levels, treatment with Rocaltrol reduces tubular excretion of phosphate and in combination with phosphorus medication normalizes bone development.
Treatment with Rocaltrol also helps with other forms of rickets, such as those caused by neonatal hepatitis, biliary atresia, cystinosis, and alimentary calcium and vitamin D deficiencies.
Pharmacokinetics
Intake
Calcitriol is rapidly absorbed in the intestine. After a single oral administration of 0.25-1.0 mcg of Rocaltrol, maximum serum concentrations are reached after 3-6 hours. With repeated administration, equilibrium concentrations are reached within 7 days and depend on the dose amount.
Distribution
After a single oral dose of 0.5 µg of Rocaltrol, the average serum calcitriol concentration rose from a baseline value of 40.0 4.4 pg/ml to 60.0 4.4 pg/ml after 2 hours and then dropped to 53.0 6.9 after 4 hours, to 50.0 7.0 after 8 hours, to 44 4.6 after 12 hours, and 41.5 5.1 pg/ml after 24 hours.
In the blood, calcitriol and other vitamin D metabolites bind to specific plasma proteins.
Exogenous calcitriol can be assumed to pass through the placental barrier and into breast milk.
Metabolism
Several calcitriol metabolites have been identified, each with different vitamin D properties: 1α,25-dihydroxy-24-oxocholecalciferol, 1α,23,25-trihydroxy-24-oxocholecalciferol, 1α,24R,25-trihydroxycholecalciferol, 1α,25R-dihydroxycholecalciferol-26,23S-lactone, 1α,25S,26-trihydroxy-cholecalciferol, 1α,25-dihydroxy-23-oxocholecalciferol, 1α,25R,26-trihydroxy-23-oxocholecalciferol and 1α-hydroxy-23-carboxy-24,25,26,27-tetranorocholecalciferol.
Elimation
The half-life of calcitriol from serum is 9-10 hours. However, the pharmacological action of a single dose of calcitriol lasts at least 7 days. Calcitriol is excreted with bile and undergoes enterohepatic circulation. After intravenous administration of labeled calcitriol healthy volunteers within 24 hours, about 27% of radioactivity is detected in the feces and about 7% – in the urine. After oral administration of 1 µg of labeled calcitriol to healthy volunteers, about 10% of total radioactivity is detectable in the urine within 24 hours. On day 6 after intravenous injection of labeled calcitriol, 16% of total radioactivity is detected in the urine and 49% of total radioactivity is detected in the feces.
Pharmacokinetics in special cases
In patients with nephrotic syndrome or in patients on hemodialysis, serum calcitriol levels are reduced and reaching maximum concentration is somewhat delayed.
Indications
Indications
Confirmed postmenopausal osteoporosis;
renal osteodystrophy in patients with chronic renal failure, especially those on hemodialysis;
secondary hyperparathyroidism in patients with moderate and severe chronic renal failure (pre-dialysis);
postoperative hypoparathyroidism;
idiopathic hypoparathyroidism;
pseudohypoparathyroidism;
vitamin D-dependent rickets;
hypophosphatemic vitamin D-resistant rickets (phosphate diabetes).
Pharmacological effect
Pharmacological effect
Rocaltrol – regulates calcium metabolism, stimulates osteogenesis, normalizes calcium content in bone tissue.
Calcitriol is one of the most important active metabolites of vitamin D 3 . Calcitriol promotes calcium absorption in the intestine and regulates bone mineralization. The pharmacological effect of a single dose of calcitriol lasts 3-5 days.
The key role of calcitriol in the regulation of calcium metabolism, consisting in stimulating the activity of skeletal osteoblasts, represents a reliable pharmacological basis for its therapeutic effect in osteoporosis.
In individuals with severe renal failure, the synthesis of endogenous calcitriol is reduced or may even stop altogether. Calcitriol deficiency plays a major role in the occurrence of renal osteodystrophy.
In patients with renal osteodystrophy, Rocaltrol (synthetic calcitriol) normalizes reduced calcium absorption in the intestine, eliminates hypocalcemia, elevated levels of alkaline phosphatase and parathyroid hormone in the serum. It reduces pain in bones and muscles, and also eliminates the histological changes that occur with osteitis fibrosa and other mineralization disorders.
In patients with postoperative and idiopathic hypoparathyroidism and pseudohypoparathyroidism, Rocaltrol reduces hypocalcemia and its clinical manifestations.
In patients with vitamin D-dependent rickets, serum calcitriol concentrations are low or completely absent. Considering the insufficient endogenous synthesis of calcitriol in the kidneys, taking Rocaltrol can be considered in such patients as replacement therapy.
In patients with vitamin D-resistant rickets and hypophosphatemia with low plasma calcitriol levels, treatment with Rocaltrol reduces tubular excretion of phosphates and, in combination with phosphorus supplements, normalizes bone development.
Treatment with Rocaltrol also helps with other forms of rickets, for example those caused by neonatal hepatitis, biliary atresia, cystinosis, as well as nutritional deficiency of calcium and vitamin D.
Pharmacokinetics
Suction
Calcitriol is rapidly absorbed in the intestine. After a single oral dose of 0.25-1.0 mcg of Rocaltrol, maximum serum concentration is achieved after 3-6 hours. With repeated administration, equilibrium concentrations are achieved within 7 days and depend on the dose.
Distribution
After a single oral dose of 0.5 mcg Rocaltrol, the mean serum calcitriol concentration rose from a baseline value of 40.0 4.4 pkg/ml to 60.0 4.4 pkg/ml after 2 hours, and then fell to 53.0 6.9 after 4 hours, to 50.0 7.0 after 8 hours, to 44 4.6 after 12 hours and 41.5 5.1 pkg/ml after 24 hours.
In the blood, calcitriol and other vitamin D metabolites bind to specific plasma proteins.
It can be assumed that exogenous calcitriol penetrates the placental barrier and into breast milk.
Metabolism
Several metabolites of calcitriol have been identified, each with different vitamin D properties: 1α,25-dihydroxy-24-oxocholecalciferol, 1α,23,25-trihydroxy-24-oxocholecalciferol, 1α,24R,25-trihydroxycholecalciferol, 1α,25R-dihydroxycholecalciferol-26,23S-lactone, 1α,25S,26-tri-hydroxycholecalciferol, 1α,25-dihydroxy-23-oxocholecalciferol, 1α,25R,26-trihydroxy-23-oxocholecalciferol and 1α-hydroxy-23-carboxy-24,25,26,27-tetranorcholecalciferol.
Removal
The serum half-life of calcitriol is 9-10 hours. However, the pharmacological effect of a single dose of calcitriol lasts at least 7 days. Calcitriol is excreted in the bile and undergoes enterohepatic circulation. After intravenous administration of labeled calcitriol to healthy volunteers within 24 hours, about 27% of the radioactivity is detected in the feces and about 7% in the urine. Following oral administration of 1 μg of labeled calcitriol to healthy volunteers, approximately 10% of the total radioactivity is detectable in urine within 24 hours. On the 6th day after intravenous administration of labeled calcitriol, 16% of total radioactivity was detected in urine, and 49% of total radioactivity in feces.
Pharmacokinetics in special cases
In patients with nephrotic syndrome or in patients on hemodialysis, serum calcitriol levels are reduced and the achievement of maximum concentrations is somewhat delayed.
Special instructions
Special instructions
There is a strong association between calcitriol treatment and the occurrence of hypercalcemia. Hypercalcemia can develop when calcium intake increases in the body due to dietary changes (for example, increased consumption of dairy products) or uncontrolled intake of calcium supplements. Patients and their families should be informed that strict adherence to the prescribed diet is mandatory; they should also be trained to recognize the symptoms of hypercalcemia.
Patients who have been on bed rest for a long time, for example, those who have undergone surgery, are at a particularly high risk of developing hypercalcemia.
Calcitriol increases the content of inorganic phosphates in serum. This effect, while desirable in patients with hypophosphatemia, requires caution in patients with renal failure due to the risk of ectopic calcification. In such cases, plasma phosphate levels should be maintained at normal levels (2-5 mg/100 ml, or 0.65-1.62 mmol/L) by oral administration of phosphate binders and a low-phosphate diet.
Patients with vitamin D-resistant rickets (familial hypophosphatemia) receiving Rocaltrol should continue to take oral phosphate. However, one should be aware of the stimulation of intestinal absorption of phosphates by Rocaltrol, since this effect may change the need for additional administration of phosphates. It is necessary to regularly determine the content of calcium, phosphorus, magnesium and alkaline phosphatase in serum, as well as calcium and phosphates in daily urine. During the stabilizing (initial) phase of treatment with Rocaltrol, calcium levels in the blood serum should be monitored at least twice a week.
Since calcitriol is the most effective metabolite of vitamin D of all existing ones, other vitamin D preparations should not be prescribed during treatment with Rocaltrol in order to avoid the development of hypervitaminosis D.
If a patient is switched from ergocalciferol (vitamin D2) to calcitriol, normalization of ergocalciferol levels in the blood may take several months (see “Overdose”).
Patients with normal renal function taking Rocaltrol should avoid dehydration by ensuring adequate fluid intake.
Particular care must be taken when prescribing the drug to children under 18 years of age.
Active ingredient
Active ingredient
Calcitriol
Composition
Composition
Active ingredient:
calcitriol0.25 mcg;
Excipients:
butylated hydroxyanisole;
butylated hydroxytoluene;
medium chain triglycerides;
Capsule shell:
gelatin; glycerol (glycerin) 85%; Karion 83 (contains 2–4% mannitol, 27–35% sorbitol, 61–71% hydrolyzed hydrogenated starch); titanium dioxide (E171); red iron oxide dye (E172); iron oxide yellow dye (E172)
Pregnancy
Pregnancy
When sublethal doses of vitamin D were administered orally to pregnant rabbits, the fetuses developed supravalvular aortic stenosis. There is no data on the teratogenicity of vitamin D, even in very large doses, in humans.
Rocaltrol should be prescribed to pregnant women only according to absolute indications, if the expected effect for the mother exceeds the possible risk to the fetus.
Apparently, exogenous calcitriol passes into breast milk. Taking into account possible hypercalcemia in the mother and adverse reactions in infants, taking the drug during breastfeeding is not recommended.
Contraindications
Contraindications
All diseases accompanied by hypercalcemia; hypersensitivity to the drug (or to drugs of the same class) or its excipients.
With caution: atherosclerosis, pulmonary tuberculosis, heart failure, phosphate nephrolithiasis, sarcoidosis and other granulomatosis, old age (may contribute to the development of atherosclerosis). Lactation period.
Side Effects
Side Effects
Since Rocaltrol® has the activity of vitamin D, its side effects are similar to those that occur with an overdose of vitamin D, i.e. hypercalcemia syndrome or calcium intoxication (depending on the severity and duration of hypercalcemia). Acute symptoms may include anorexia, headache, vomiting, gastralgia or abdominal pain and constipation. Due to the short biological half-life of calcitriol, serum calcium levels return to normal within a few days after discontinuation of Rocaltrol®, much more quickly than with treatment with vitamin D3 supplements.
With long-term use, dystrophy, sensitivity disorders, fever, thirst, polyuria, dehydration, apathy, growth retardation and urinary tract infections may occur.
The frequency of each of the adverse reactions described during the clinical use of Rocaltrol® for all indications over 15 years is very low (including hypercalcemia) and does not exceed 0.001%.
With simultaneous hypercalcemia and hyperphosphatemia >6 mg/100 ml or >1.9 mmol/L, soft tissue calcification may occur, which can be detected radiographically.
In patients with normal renal function, chronic hypercalcemia may lead to elevated serum creatinine.
Sensitive individuals may develop hypersensitivity reactions (itching, rash, urticaria and very rarely erythematous skin lesions).
Interaction
Interaction
Since calcitriol is one of the most important active metabolites of vitamin D3, in order to avoid possible additive effects and hypercalcemia, dosage doses of vitamin D and its derivatives should be discontinued during treatment with Rocaltrol.
Patients should strictly follow dietary recommendations, especially regarding calcium intake, and also avoid uncontrolled additional intake of drugs containing calcium.
Concomitant treatment with thiazide diuretics increases the risk of hypercalcemia. In patients receiving digitalis preparations, the dose of calcitriol should be selected very carefully, since hypercalcemia in them can provoke arrhythmias.
There is functional antagonism between vitamin D analogues, which enhance calcium absorption, and corticosteroids, which inhibit it.
Preparations containing magnesium (for example, antacids) can cause hypermagnesemia and therefore should not be prescribed to patients on chronic hemodialysis during treatment with Rocaltrol.
Since Rocaltrol affects the transport of phosphate in the intestine, kidneys and bones, the dose of phosphate binders should be adjusted depending on the level of phosphate in the serum (normal – 2-5 mg/100 ml, or 0.65-1.62 mmol/l).
The use of enzyme inducers such as phenytoin or phenobarbital may increase metabolism and thereby decrease serum calcitriol concentrations, so higher doses of calcitriol may be required when these drugs are used concomitantly.
Cholestyramine may reduce the intestinal absorption of fat-soluble vitamins, including calcitriol.
Overdose
Overdose
Treatment of asymptomatic hypercalcemia (see section “Dosage and Administration”).
Since Rocaltrol® is a vitamin D derivative, it is characterized by the same symptoms of overdose. Taking large doses of calcium and phosphate at the same time as Rocaltrol® may cause similar symptoms. The product of serum calcium and phosphorus concentrations (CaxP) should not exceed 70 mg2/dl2. The development of hypercalcemia may be promoted by high calcium levels in the dialysate.
Symptoms of acute vitamin D poisoning: anorexia, headache, vomiting, constipation.
Symptoms of chronic poisoning: dystrophy (weakness, weight loss), sensitivity disorders, possible fever with thirst, polyuria, dehydration, apathy, growth retardation and urinary tract infections. The consequences of hypercalcemia are focal calcification of the renal cortex, myocardium, lungs and pancreas.
Treatment of accidental overdose: Immediate gastric lavage or administration of anti-vomiting agents to prevent further absorption. To remove the drug with feces, Vaseline oil is used as a laxative. It is recommended to repeat serum calcium levels. If elevated serum calcium levels persist, phosphate and corticosteroids may be prescribed and measures taken to ensure adequate diuresis.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
F.Hoffmann-La Roche Ltd, Switzerland
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | F. Hoffmann-La Roche Ltd, Switzerland |
| Medication form | capsules |
| Brand | F. Hoffmann-La Roche Ltd |
Related products
Buy Rocaltrol, 0.25 mcg capsules 30 pcs with delivery to USA, UK, Europe and over 120 other countries.