No products in the cart.
Ripart Long 20 mg/ml 2 ml syringe
€1.00
Out of stock
(E-mail when Stock is available)
Description
– Osteoarthritis and other degenerative-dystrophic, traumatic and post-traumatic changes of joints: knees, hips and other joints.
– As an aid in orthopedic surgery.
– For use in patients with increased physical activity and who regularly load the damaged joint.
Active ingredient
Active ingredient
Composition
Composition
1ml contains:
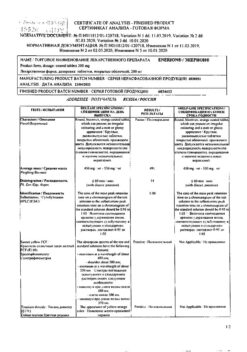
Sodium hyaluronate 20 mg
Sodium dihydrophosphate 0.1 mg
Dinatrium hydrophosphate 0.6 mg
Sodium chloride, 0.9% solution for infusion – up to the volume of 1 ml.
How to take, the dosage
How to take, the dosage
The amount of Synovial Fluid Replacement is dependent on the size of the joint. The physician is responsible for determining the applicable volume and must ensure that the joint is not overloaded.
The treatment with RIPART® Long Synovial Fluid Replacement Solution usually requires a cycle of 1-2 injections (1 week apart).
The choice of dosage is left to the discretion of the treating physician.
The course may be repeated if necessary.
The decision about whether to repeat a course is at the discretion of the physician.
How to use the product
Inject the required volume of Synovial Fluid Replacement Agent into each joint. If there is a doctor’s order to give in more than one joint, a separate syringe should be used for each joint.
The Synovial Fluid Replacement Product is a SINGLE MEDICAL USE (DO NOT REQUIRE USE).
Special Instructions
Special Instructions
Cautions
Risks of Use
The manufacturer has defined the risks to the patient associated with the occurrence of each type of hazard and the occurrence of their consequences. The parameters of risk reduction are calculated with the application of measures to prevent conditions for the occurrence of hazards or the occurrence of their consequences.
It is established that no new risks arise after the implementation of risk control measures, and the overall residual risk is recognized as acceptable; the risks in manufacturing do not change from the composition and type of packaging of the medical device.
Patient Information
Serility
The product is supplied sterile. Sterilization parameters: steam sterilization method according to GOST R ISO 17665-1.
Re-sterilization of the product is prohibited. Re-use is prohibited.
Biodegradation of the medical device in the patient
The protective film formed after the injection of the Synovial Fluid Substitute retains its elastic properties for 6 months.
After six months, Synovial Fluid Replacement breaks down under the action of a group of tissue enzymes called hyaluronidases into breakdown products: oligosaccharides and low-molecular-weight hyaluronates, which are then eliminated from the body naturally by metabolic processes.
The Synovial Fluid Substitute cannot be removed or replaced as it is inseparably mixed with the synovial fluid of the joint.
Contraindications
Contraindications
Side effects
Side effects
Similarities
Similarities
Additional information
| Conditions of storage | The medical device is used indoors at an ambient temperature of 2°C to 25°C. The product must be transported by all means of transportation, in closed vehicles, in accordance with the cargo regulations applicable to the mode of transportation. The Synovial Fluid Substitute must be transported in the original manufacturer's packaging at 2°C to 25°C and at a relative humidity of 30 to 75%, with no condensation. The Synovial Fluid Replacement Product must be stored in the original packaging, protected from direct sunlight, heat and freezing, at 2°C to 25°C and at 30 to 75% non-condensing relative humidity. |
|---|---|
| Manufacturer | Ingal Ltd, Russia |
| Medication form | solution for injection |
| Brand | Ingal Ltd |
Related products
Buy Ripart Long 20 mg/ml 2 ml syringe with delivery to USA, UK, Europe and over 120 other countries.