No products in the cart.
Rigavidon 21+7, 0.15 mg+0.03 mg 28 pcs.
€12.89 €11.28
Out of stock
(E-mail when Stock is available)
EAN: 5997001359068
SKU: 207290
Categories: Contraceptive, Gynecology and Obstetrics, Hormonal, Medicine
Description
Pharmacodynamics
Rigevidone is an oral monophasic combined estrogen-gestagen contraceptive.
When taken orally, it inhibits pituitary gonadotropic hormone secretion.
The contraceptive effect is associated with several mechanisms. As a gestagenic component (progestin) contains a derivative of 19-nortestosterone – levonorgestrel, which is superior in activity to the corpus luteum hormone progesterone (and the synthetic analog of the latter – pregnin), acts at the level of receptors without prior metabolic transformations. The estrogenic component is ethinyl estradiol.
Under the influence of levonorgestrel there is a blockade of the release of releasing rhysing hormones (LH and FSH) of the hypothalamus, inhibition of secretion of gonadotropic hormones by the pituitary gland, which leads to inhibition of maturation and release of the egg ready for fertilization (ovulation). Contraceptive effect is enhanced by ethinyl estradiol. Maintains high viscosity of cervical mucus (makes it difficult for sperm to enter the uterine cavity). In addition to the contraceptive effect of regular use normalizes the menstrual cycle and contributes to the prevention of a number of gynecological diseases, including the tumor nature.
Pharmacokinetics
Levonorgestrel is quickly absorbed (less than 4 hours). Levonorgestrel has no effect of first passage through the liver. When levonorgestrel is coadministered with ethinylestradiol, there is a dose-response relationship with maximum plasma concentration. TCmax (time of reaching maximum concentration) of levonorgestrel is 2 hours, T1/2 (elimination half-life) is 8-30 hours (average 16 hours). (on average 16 hours). Most of the levonorgestrel is bound in the blood with albumin and hGH (sex hormone-binding globulin).
Ethinylestradiol is quickly and almost completely absorbed from the intestine. Ethinylestradiol has an inherent effect of primary passage through the liver, TCmax is 1.5 hours, and the elimination half-life is about 26 hours.
When administered orally, ethinylestradiol is excreted from blood plasma within 12 hours, the half-life is 5.8 hours.
Metabolism of ethinylestradiol is carried out in the liver and intestine. Metabolites of ethinylestradiol are water-soluble products of sulfate or glucuronide conjugation and enter the intestine with bile, where they are disintegrated by intestinal bacteria.
The two components (levonorgestrel and ethinylestradiol) are excreted with breast milk. The active substances are metabolized in the liver, the T1/2 is 2-7 hours.
Levonorgestrel is excreted by the kidneys (60%) and through the intestine (40%); ethinylestradiol by the kidneys (40%) and through the intestine (60%).
Indications
Indications
Oral contraception, functional menstrual cycle disorders (including dysmenorrhea without an organic cause, dysfunctional metrorrhagia, premenstrual syndrome).
Active ingredient
Active ingredient
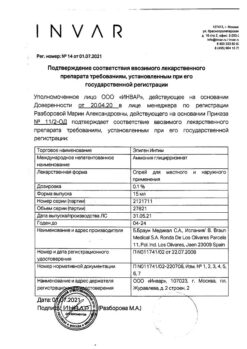
Composition
Composition
Tablets ethinylestradiol + levonorgestrel
1 tablet contains:
active ingredients:
ethinylestradiol 0.03 mg,
levonorgestrel 0.15 mg,
excipients:
core:
Silica colloidal, 0.275 mg; magnesium stearate, 0.55 mg; talc, 1.1 mg; Corn starch – 19.895 mg; lactose monohydrate – 33 mg
coating:
sucrose, 22.459 mg; talc, 6.826 mg; calcium carbonate, 3.006 mg; titanium dioxide, 1.706 mg; copovidone, 0.592 mg; macrogol 6000, 0.148 mg; colloidal silicon dioxide, 0.146 mg; povidone, 0.088 mg; sodium carmellose, 0.029 mg
Tablets placebo iron
1 tablet contains:
core:
Iron fumarate, 76.05 mg; colloidal silicon dioxide, 0.5 mg; croscarmellose sodium, 0.6 mg; magnesium stearate, 1.2 mg; povidone, 2.4 mg; Talc, 2.4 mg; potato starch, 2.1 mg; corn starch, 10.2 mg; lactose monohydrate, 24.55 mg
coating:
sucrose, 38.295 mg; talc, 11.752 mg; calcium carbonate, 5.103 mg; titanium dioxide, 2.226 mg; copolyvidone, 1.124 mg; Colloidal silica – 0.24 mg; red iron oxide (E172) – 0.787 mg; povidone – 0.144 mg; macrogol 6000 – 0.281 mg; sodium carmellose – 0.048 mg
How to take, the dosage
How to take, the dosage
Ingestion. 1 tablet a day, preferably at the same time of the day.
If a woman has not taken a contraceptive in her previous cycle and the doctor has not prescribed otherwise: the 1st tablet should be taken on the 1st day of menstruation, and the pills should be continued for 21 days. Then it is recommended to continue taking the reddish-brown pills for 7 days, during which menstrual bleeding occurs. After that, you should continue taking the next package containing 21 white tablets and then 7 reddish-brown tablets – without a break. Each cycle of taking begins on the same day of the week.
If a woman took the contraceptive in a previous cycle and the previous packet contained 21 pills: you must start taking the drug after a 7-day break, on the 8th day.
The composition of the pills of different colors is not the same. Therefore, the beginning and the correct sequence of taking – 21 white pills first, followed by 7 reddish-brown pills – are indicated by numbers and arrows on the package.
If you switch to Rigevidone® 21+7 from another contraceptive, the above chart should be used.
After childbirth or abortion: you may not start until the 1st day of your period of the 1st biphasic cycle. The 1st biphasic cycle is usually shortened due to premature ovulation. If the drug is started as early as the 1st spontaneous bleeding, the drug cannot successfully prevent premature ovulation, so contraception may not be reliable in the first 2 weeks of the cycle.
If the pill is missed within the prescribed period: the missed pill should be taken within the next 12 hours. In this case there is no need to use additional contraceptive methods. It is recommended to take the remaining tablets at the usual time. If more than 12 hours have elapsed, the last missed pill should be taken (skipping the other unused pills) and the drug should be taken normally. In this case, additional contraceptive methods (barrier methods, spermicides) should be used for the next 7 days.
This does not apply to the reddish-brown pills, since they do not contain hormones.
For therapeutic purposes: the dose of the drug and the route of administration are chosen by the doctor for each patient individually.
Interaction
Interaction
Barbiturates, some antiepileptic drugs (carbamazepine, phenytoin), sulfonamides, pyrazolone derivatives can increase the metabolism of the steroid hormones included in the drug.
Lack of contraceptive effectiveness may also be observed when concomitantly prescribed with some antimicrobials (including ampicillin, rifampicin, chloramphenicol, neomycin, polymyxin B, sulfonamides, tetracyclines), which is associated with changes in the intestinal microflora.
In concomitant use with anticoagulants, coumarin or indandion derivatives, additional determination of the prothrombin index and change of the anticoagulant dose may be required.
The use of tricyclic antidepressants, maprotiline, beta-adrenoblockers may increase their bioavailability and toxicity.
When using oral hypoglycemic agents and insulin, it may be necessary to change their dosage.
When combined with bromocriptine its effectiveness decreases.
When combined with drugs with potential hepatotoxic effects, such as the drug dantrolene, an increase in hepatotoxicity is observed, especially in women over 35 years.
Rigevidone should be used with caution in combination with the drugs listed above.
Special Instructions
Special Instructions
Before starting hormonal contraception and every 6 months thereafter, a general medical and gynecological examination is recommended, including cervical smear cytology, assessment of mammary glands, determination of blood glucose, cholesterol and other liver function parameters, BP control, urine analysis).
Prescribing Rigevidone to women with thromboembolic diseases at a young age and a family history of increased blood clotting is not recommended.
The use of oral contraception is allowed no earlier than 6 months after having had viral hepatitis on condition of normalization of liver functions.
In case of severe upper abdominal pain, hepatomegaly, and signs of intra-abdominal bleeding, liver tumor may be suspected. If necessary, the drug should be discontinued.
In case of impaired liver function while taking Rigevidone, a consultation with a general practitioner is necessary.
If acyclic (intermenstrual) bleeding occurs, Rigevidone should be continued, as most of these bleeding stops spontaneously. If acyclical (intermenstrual) bleeding persists or recurs, a medical exam should be performed to rule out an organic reproductive system disorder.
If vomiting or diarrhea occurs, the drug should be continued with another, non-hormonal method of contraception.
Women who smoke and take hormonal contraceptives have an increased risk of cardiovascular disease with serious consequences (myocardial infarction, stroke). The risk increases with age and according to the number of cigarettes smoked (especially in women over the age of 35).
The drug should be discontinued in the following cases:
– If a migraine-like headache occurs for the first time or worsens.
– If an unusually severe headache occurs.
– If there are early signs of phlebitis or phlebothrombosis (unusual pain or swollen veins in the legs).
– In the occurrence of jaundice or hepatitis without jaundice.
– In cerebrovascular disorders.
– In the occurrence of stabbing pain of unclear etiology when breathing or coughing, pain and feeling of tightness in the chest.
– In acute deterioration of visual acuity.
– In suspected thrombosis or heart attack.
– In sudden increase in BP.
– In the occurrence of generalized itching.
– In the frequency of epileptic seizures.
– In 3 months before the planned pregnancy.
– Approximately 6 weeks before the planned surgical intervention.
– In case of prolonged immobilization.
– In case of a pregnancy.
– Application in liver function disorders: application is contraindicated in severe liver diseases (including congenital hyperbilirubinemia – Gilbert, Dubin-Johnson and Rotor syndromes; liver tumors).
– Administration in renal dysfunction: Caution is required if it is necessary to prescribe the drug in patients with renal dysfunction.
Impact on driving and operating machinery: Administration of the drug does not affect the ability to drive vehicles and operate other mechanisms with increased risk of injury.
Contraindications
Contraindications
– Severe liver diseases (including congenital hyperbilirubinemia – Gilbert, Dubin-Johnson and Rotor syndromes).
– Cholecystitis.
– Presence or history of severe cardiovascular and cerebrovascular diseases.
– Thromboembolism and predisposition to it.
– Malignant tumors (especially breast or endometrial cancer).
– Liver tumors.
– Familial forms of hyperlipidemia.
– Severe forms of arterial hypertension.
– Sickle-cell anemia.
– Chronic hemolytic anemia.
– Vaginal bleeding of unknown etiology.
– Bubble drift.
– Migraine.
– Otosclerosis.
– A history of idiopathic jaundice in pregnant women.
– Severe skin itching in pregnant women.
– Pregnant herpes.
– Age over 40 years.
– Pregnancy.
– Lactation period (breastfeeding).
– Hypersensitivity to the components of the drug.
The drug should be used with caution:
– Diseases of the liver and gallbladder.
– Epilepsy.
– Depression.
– Ulcerative colitis.
– Uterine myoma.
– Mastopathy.
– Tuberculosis.
– Kidney diseases.
– Diabetes.
– Cardiovascular diseases.
– Arterial hypertension.
– Kidney function disorders.
– Varicose veins.
– Phlebitis.
– Multiple sclerosis.
– Minor chorea.
– In adolescence (without regular ovulatory cycles).
Side effects
Side effects
The drug is usually well tolerated.
Possible transient, spontaneous side effects include: nausea, vomiting, headache, breast engorgement, weight and libido changes, mood changes, acyclic bloody discharge, and, in some cases, eyelid edema, conjunctivitis, visual disturbances, discomfort while wearing contact lenses (these phenomena are temporary and disappear after withdrawal without any therapy).
Long-term use may very rarely result in chloasma, hearing loss, generalized pruritus, jaundice, calf cramps, increased frequency of epileptic seizures. Hypertriglyceridemia, hyperglycemia, decreased glucose tolerance, increased arterial pressure (BP), thrombosis and venous thromboembolism, jaundice, skin rash, change of vaginal secretion pattern, vaginal candidiasis, increased fatigue, diarrhea are rarely observed.
Overdose
Overdose
There are no known cases of toxic effects due to overdose.
Pregnancy use
Pregnancy use
The drug is contraindicated in pregnancy and during lactation (breastfeeding).
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At 15-30 °C |
| Manufacturer | Gedeon Richter, Hungary |
| Medication form | pills |
| Brand | Gedeon Richter |
Related products
Gynecology and Obstetrics
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Buy Rigavidon 21+7, 0.15 mg+0.03 mg 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.