No products in the cart.
Resorba, lyophilizate 4 mg 5 ml
€1.00
Out of stock
(E-mail when Stock is available)
Description
The drug Resorba belongs to a new class of highly effective bisphosphonates with a selective effect on bone tissue. It suppresses the activity of osteoclasts and has no undesirable effect on the formation, mineralization and mechanical properties of bone tissue. The selective action of bisphosphonates on bone tissue is based on their high affinity for mineralized bone tissue, but the exact molecular mechanism providing inhibition of osteoclast activity is still unknown. It also has direct antitumor properties that provide efficacy in bone metastases.
In vitro it was found that zoledronic acid, by inhibiting cell proliferation and inducing cell apoptosis, has a direct antitumor effect on myeloma and breast cancer cells, reducing their risk of metastasis. Inhibition of osteoclastic bone resorption, which changes bone marrow microenvironment, leads to reduction of tumor cell growth; anti-angiogenic and anti-tumor activity is noted.
Zoledronic acid also inhibits proliferation of human endothelial cells. In tumor-induced hypercalcemia, it reduces serum calcium concentration.
Pharmacokinetics
Pharmacokinetic parameters are not dose dependent. After initiation of infusion, serum concentrations increase rapidly, reaching Cmax at the end of infusion, followed by a rapid decrease in concentration: by 10% after 4 h and to less than 1% of Cmax after 24 h with a further prolonged period of low concentrations, not exceeding 0.1% of Cmax, until repeat infusion on day 28.
The binding to plasma proteins is 56%. It is not metabolized.
It is excreted unchanged by the kidneys in 3 stages: 1st and 2nd stage – rapid elimination of the drug from systemic blood flow, with T1/2 – 0.24 h and 1.87 h, respectively, and the 3rd stage – prolonged, with T1/2 – 146 h. No drug cumulation was observed in repeated injections every 28 days. During the first 24 h, 23-55% is detectable in the urine.
The rest of the drug is bound to the bone tissue, after which it is slowly re-released into the systemic bloodstream and excreted by the kidneys; less than 3% is excreted with the feces. Total plasma clearance is 2.54-7.54 l/h. It does not depend on the drug dose, sex, age, race and body weight of the patient. Increasing the duration of infusion from 5 to 15 min leads to a 30% decrease in zoledronic acid concentration at the end of infusion, but does not affect the AUC.
Renal clearance correlates positively with creatinine Cl and is 42-108% of Cl creatinine averaging 55-113%. In patients with severe (creatinine Cl ≤20 ml/min) and moderate renal insufficiency (creatinine Cl from 20 to 50 ml/min) clearance of zoledronic acid is 37 and 72%, respectively, from the drug clearance values in patients with creatinine Cl ≥84 ml/min.
Indications
Indications
Hypercalcemia (albumin-corrected serum calcium concentration ≥12 mg/dL or 3 mmol/L) induced by malignant tumors; metastatic bone lesions from solid malignancies and multiple myeloma (to reduce the risk of pathological fractures, spinal cord compression, tumor-related hypercalcemia, and reduce the need for radiation therapy).
Pharmacological effect
Pharmacological effect
Rezorba belongs to a new class of highly effective bisphosphonates that have a selective effect on bone tissue. Suppresses the activity of osteoclasts and does not have undesirable effects on the formation, mineralization and mechanical properties of bone tissue. The selective effect of bisphosphonates on bone tissue is based on a high affinity for mineralized bone tissue, but the exact molecular mechanism that provides inhibition of osteoclast activity remains unclear. It also has direct antitumor properties, ensuring effectiveness in bone metastases.
In vitro, it was found that zoledronic acid, by suppressing proliferation and inducing cell apoptosis, has a direct antitumor effect on myeloma and breast cancer cells and reduces the risk of their metastasis. Inhibition of osteoclastic bone resorption, which alters the bone marrow microenvironment, leads to decreased tumor cell growth; antiangiogenic and analgesic activity is noted.
Zoledronic acid also inhibits the proliferation of human endothelial cells. With hypercalcemia caused by a tumor, it reduces the concentration of calcium in the blood serum.
Pharmacokinetics
Pharmacokinetic parameters are independent of dose. After the start of the infusion, serum concentrations increase rapidly, reaching Cmax at the end of the infusion, followed by a rapid decrease in concentration: by 10% after 4 hours and to less than 1% of Cmax after 24 hours, followed by a prolonged period of low concentrations not exceeding 0.1% of Cmax, until re-infusion on the 28th day.
Communication with plasma proteins – 56%. Not metabolized.
It is excreted unchanged by the kidneys in 3 stages: the 1st and 2nd stages – rapid elimination of the drug from the systemic circulation, with T1/2 – 0.24 hours and 1.87 hours, respectively, and the 3rd stage – long-term, with T1/2 – 146 hours. No accumulation of the drug was observed with repeated administrations every 28 days. During the first 24 hours, 23–55% is found in urine.
The remaining amount of the drug binds to bone tissue, after which it is slowly released back into the systemic circulation and excreted by the kidneys; Less than 3% is excreted in feces. Total plasma clearance is 2.54–7.54 l/h. It does not depend on the dose of the drug, gender, age, race or body weight of the patient. Increasing the infusion duration from 5 to 15 minutes resulted in a 30% decrease in zoledronic acid concentration at the end of infusion but had no effect on AUC.
Renal clearance is positively correlated with Cl creatinine and amounts to 42–108% of Cl creatinine, which averages 55–113%. In patients with severe (Cl creatinine ≤20 ml/min) and moderate renal failure (Cl creatinine from 20 to 50 ml/min), the clearance of zoledronic acid is 37 and 72%, respectively, of the drug clearance in patients with Cl creatinine ≥84 ml/min.
Special instructions
Special instructions
Before infusion, ensure that the patient is adequately hydrated. If necessary, administration of saline before, during, or after the zoledronic acid infusion is recommended. Overhydration of the patient should be avoided due to the risk of cardiovascular complications.
After administration of the drug, constant monitoring of the concentrations of calcium, magnesium, phosphorus and creatinine in the blood serum is necessary.
Renal function should be closely monitored during zoledronic acid therapy. Risk factors for renal dysfunction include dehydration, previous renal failure, repeated administration of zoledronic acid or other bisphosphonates, as well as the use of nephrotoxic drugs and too rapid administration of the drug.
It should be borne in mind that cases of bronchospasm have been reported when prescribing other bisphosphonates to patients with bronchial asthma sensitive to acetylsalicylic acid, but such cases have not yet been reported with the use of zoledronic acid.
During treatment with bisphosphonates, including zoledronic acid, cases of the development of osteonecrosis of the jaw have been described in cancer patients, and therefore, before starting treatment with bisphosphonates, it is necessary to provide a dental examination and, in the case of risk factors (anemia, coagulopathies, infections, poor hygiene or oral diseases, concomitant chemotherapy or radiation therapy, treatment with corticosteroids), to carry out appropriate preventive procedures. During treatment with zoledronic acid, patients with risk factors should avoid dental surgery if possible.
Influence on the ability to drive a car and other mechanisms. Studies of the effect of the drug on the ability to drive vehicles and operate complex mechanisms have not been conducted. In case of side effects from the central nervous system, it is recommended to avoid activities that require increased attention and speed of mental and motor reactions.
Active ingredient
Active ingredient
Zoledronic acid
Composition
Composition
Active ingredient:
zoledronic acid monohydrate;
Excipients:
d-mannitol;
sodium citrate dihydrate;
Solvent in ampoule:
water for injection 5 ml;
Solvent in a polymer container:
sodium chloride 9.0 g;
water for injections.
Pregnancy
Pregnancy
Contraindicated during pregnancy. Breastfeeding should be stopped during treatment.
Contraindications
Contraindications
hypersensitivity to zoledronic acid, other bisphosphonates or any other components included in the drug;
severe renal failure (creatinine Cl less than 30 ml/min);
pregnancy and lactation;
children and adolescents (safety and effectiveness of use have not been established).
With caution: impaired renal function; severe liver failure (no data on use); patients with bronchial asthma sensitive to acetylsalicylic acid.
Side Effects
Side Effects
From the hematopoietic organs: often – anemia; sometimes – thrombocytopenia, leukopenia; rarely – pancytopenia.
From the nervous system: often – headache; sometimes – dizziness, paresthesia, taste disturbances, hypoesthesia, hyperesthesia, tremor, anxiety, sleep disorders; rarely – confusion.
From the organs of vision: often – conjunctivitis; sometimes blurred vision; very rarely – uveitis, episcleritis.
From the gastrointestinal tract: often – nausea, vomiting, anorexia; sometimes – diarrhea, constipation, abdominal pain, dyspepsia, stomatitis, dry mouth.
From the respiratory system: sometimes – shortness of breath, cough.
From the skin and skin appendages: sometimes – itching, rash (including erythematous and macular), increased sweating.
From the musculoskeletal system: often – bone pain, myalgia, arthralgia, generalized pain; sometimes – muscle cramps.
From the cardiovascular system: sometimes – a pronounced increase or decrease in blood pressure; rarely – bradycardia.
From the urinary system: often – renal dysfunction; sometimes – acute renal failure, hematuria, proteinuria.
From the immune system: sometimes – hypersensitivity reactions; rarely – angioedema.
Laboratory abnormalities: very often – hypophosphatemia; often – increased serum concentrations of creatinine and urea, hypocalcemia; sometimes – hypomagnesemia, hypokalemia; rarely – hyperkalemia, hypernatremia.
Local reactions: pain, irritation, swelling, formation of infiltrate at the site of drug administration.
Other: often – fever, flu-like syndrome (including general malaise, chills, pain, fever), sometimes – asthenia, peripheral edema; chest pain, weight gain.
Interaction
Interaction
Solutions containing calcium, in particular Ringer’s solution, should not be used as solvents.
When used simultaneously with antitumor drugs, diuretics, antibiotics, analgesics, no clinically significant interactions were observed.
Bisphosphonates and aminoglycosides have a unidirectional effect on the concentration of calcium in the blood serum, therefore, when administered simultaneously, the risk of developing hypocalcemia and hypomagnesemia increases. If hypocalcemia, hypophosphatemia or hypomagnesemia develops, short-term additional administration of the corresponding substances may be necessary. Patients with untreated hypercalcemia usually have impaired renal function, so careful monitoring of renal function in this category of patients is necessary. When deciding whether to treat patients with bone metastases with zoledronic acid, it should be taken into account that the therapeutic effect occurs 2-3 months after the start of treatment with zoledronic acid.
Caution is necessary when using zoledronic acid concomitantly with drugs that have a potential nephrotoxic effect. In patients with multiple myeloma, the risk of developing renal dysfunction may be increased when bisphosphonates are administered intravenously in combination with thalidomide. Although the risk of the above-described complications is reduced when zoledronic acid is administered at a dose of 4 mg for at least 15 minutes, the possibility of renal dysfunction remains. There have been cases of deterioration in renal function, progression of renal failure and the need for hemodialysis with the first or single use of zoledronic acid.
The drug should not be mixed with other drugs.
Overdose
Overdose
Symptoms: In case of acute overdose of zoledronic acid, renal dysfunction may occur, including renal failure, changes in electrolyte composition, including the concentration of calcium, phosphate and magnesium in the blood plasma. In case of accidental overdose, the patient should be under constant supervision.
Treatment: in case of hypocalcemia accompanied by clinical manifestations, infusions of calcium gluconate are indicated.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25 °C
Shelf life
Shelf life
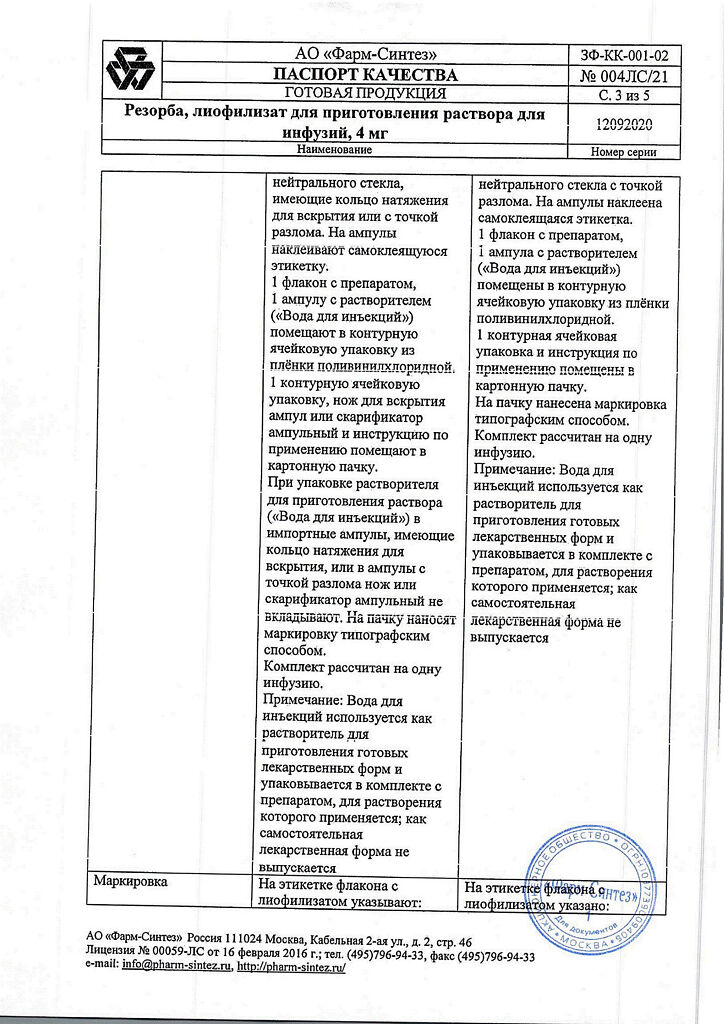
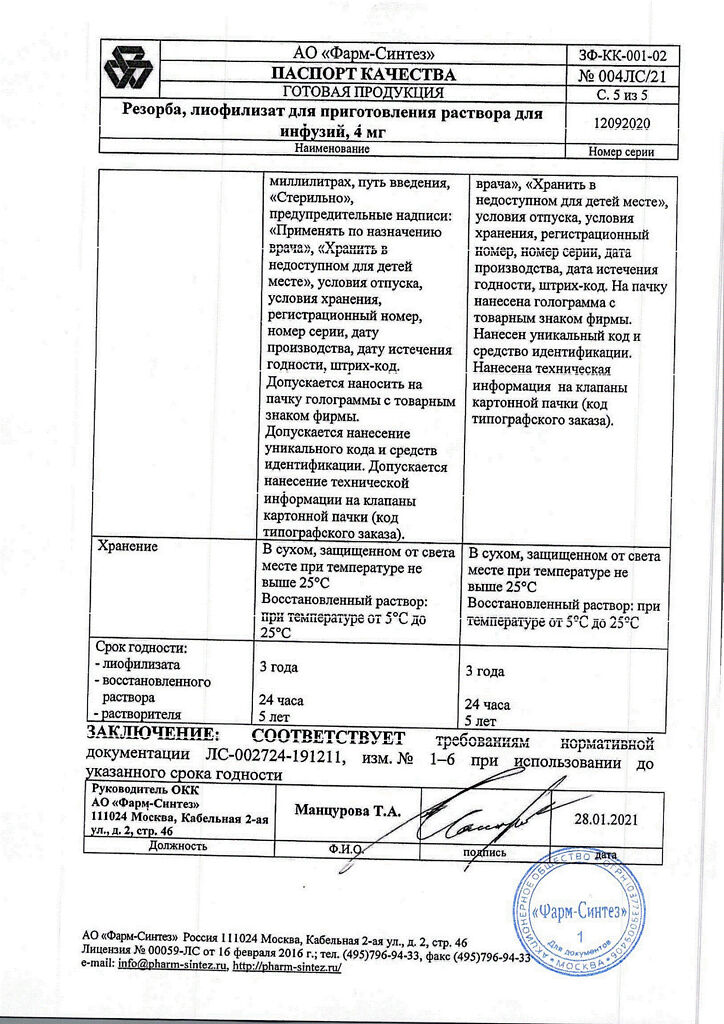
Lyophilisate – 3 years, reconstituted solution – 24 hours, solvent (water for injection) – 5 years, solvent (sodium chloride solution for infusion 0.9%) – 3 years. The expiration date of the kit is determined by the component
Manufacturer
Manufacturer
Pharm-Sintez, Russia
Additional information
| Shelf life | Lyophilizate – 3 years, reconstituted solution – 24 hours, solvent (water for injection) – 5 years, solvent (sodium chloride solution for infusion 0.9%) – 3 years. The shelf life of the kit is set by component |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25 °C |
| Manufacturer | Pharm-Sintez, Russia |
| Medication form | lyophilizate |
| Brand | Pharm-Sintez |
Related products
Buy Resorba, lyophilizate 4 mg 5 ml with delivery to USA, UK, Europe and over 120 other countries.