No products in the cart.
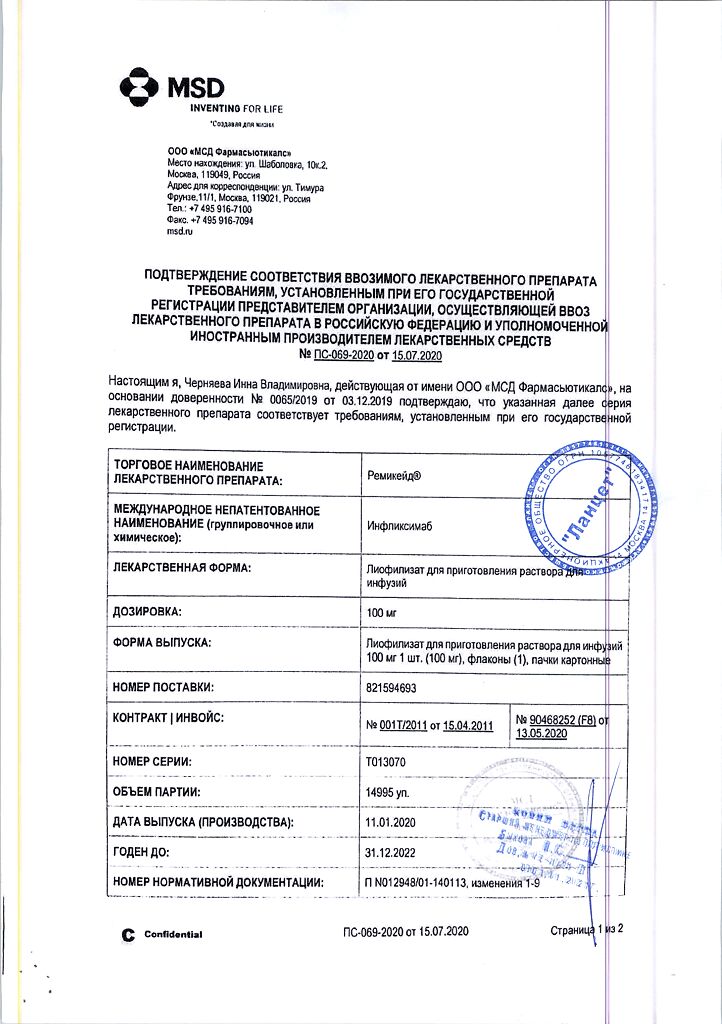
Remicade, lyophilizate 100 mg
€1.00
Out of stock
(E-mail when Stock is available)
Description
Remicade is an immunosuppressive.
Interacts with soluble and transmembrane forms of human tumor necrosis factor alpha (a cytokine of broad biological action) and reduces its functional activity, due to the formation of a stable complex.
Indications
Indications
Rheumatoid arthritis (active form), Crohn’s disease (active form).
Active ingredient
Active ingredient
Composition
Composition
Active ingredient: infliximab 100 mg.
How to take, the dosage
How to take, the dosage
Remicade is administered by IV drip, for 2 hours at a rate not exceeding 2 ml/min, using an infusion system with a built-in sterile apirogens filter with low protein-binding activity. In rheumatoid arthritis, treatment is carried out in combination with methotrexate. Initial single dose of infliximab is 3 mg/kg, after 2 and 6 weeks it is repeated in the same dose, then every 8 weeks (total number of injections – 5-6). In Crohn’s disease (severe course) – once 5 µ/kg. In Crohn’s disease with formation of fistulas – single dose – 5 mg/kg, repeatedly administered 2 and 6 weeks after the first injection. In case of disease relapse, the drug may be administered again within 14 weeks after the last dose. For preparation of infusion solution the content of the bottle shall be dissolved in 10 ml of water for injection and the total volume shall be brought to 250 ml of 0.9% NaCl solution. The resulting solution should be colorless or light yellow and opalescent; it may contain small amounts of small translucent particles (solution with dark particles or discolored is not used). Due to the absence of a preservative in the preparation, infusion of the infusion solution should be started as soon as possible and no later than 3 hours after its preparation. Unused part of the infusion solution is not subject to further use.
Interaction
Interaction
In patients with rheumatoid arthritis and Crohn’s disease, concomitant use of methotrexate or other immunomodulators reduces antibody formation to Remicade and increases its plasma concentrations.
The GKS have almost no effect on the pharmacokinetics of Remicade.
Combining treatment with Remicade and anakinra is not recommended.
There are no data on interactions between infliximab and other drugs.
Pharmaceutical Interactions
Remicaide solution should not be mixed with other medications during infusion.
Special Instructions
Special Instructions
Repeat treatment with Remicade at intervals longer than 15 weeks is not recommended; women of reproductive age should use reliable contraceptive methods during treatment with Remicade and for 6 months thereafter
Contraindications
Contraindications
Hypersensitivity (including to other murine proteins), sepsis, clinically expressed infectious disease or abscess, pregnancy, breastfeeding.
Side effects
Side effects
Nervous system disorders: headache, dizziness, fainting, depression, psychosis, anxiety, amnesia, apathy, nervousness, drowsiness.
Systemic diseases: blood flushing to the skin, increased or decreased BP, petechiae, thrombophlebitis, bradycardia, palpitations, vasospasm, cyanosis, peripheral circulatory disorders, arrhythmia.
Digestive system disorders: nausea, diarrhea, abdominal pain, dyspepsia, constipation, gastroesophageal reflux, cheilitis, diverticulitis, liver dysfunction, cholecystitis.
Urinary system disorders: urinary tract infections, pyelonephritis.
Urinary system disorders: vaginitis.
Musculoskeletal system: myalgia, arthralgia.
Hematopoietic disorders: anemia, leukopenia, lymphoadenopathy, lymphocytosis, lymphopenia, neutropenia, thrombocytopenia.
Respiratory system: upper respiratory tract infections, bronchitis, pneumonia, shortness of breath, sinusitis, nasal bleeding, bronchospasm, pleurisy.
Sensory organs: conjunctivitis, keratoconjunctivitis, endophthalmitis.
Skin and subcutaneous fatty tissue disorders: urticaria, increased sweating, dry skin, eczema, seborrhea, periorbital edema, hyperkeratosis, skin pigmentation disorders, alopecia, bullous rash.
Allergic reactions (delayed type): myalgia and/or arthralgia with fever, urticaria, itching, swelling of the face, lips, hands, dysphagia, headache.
Other: formation of autoantibodies, lupus syndrome, excessive fatigue, chest pain, edema, infusion syndrome; viral infections (influenza, herpes), abscess, sepsis, bacterial and fungal infections (rye, warts, furunculosis, onychomycosis, phlegmon).
Pregnancy use
Pregnancy use
Remicade is contraindicated in pregnancy, lactation, and children under 17 years of age
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At 2-8 °C (do not freeze) |
| Manufacturer | MSD International GmbH (Singapore Branch), Singapore |
| Medication form | lyophilizate |
| Brand | MSD International GmbH (Singapore Branch) |
Related products
Buy Remicade, lyophilizate 100 mg with delivery to USA, UK, Europe and over 120 other countries.