No products in the cart.
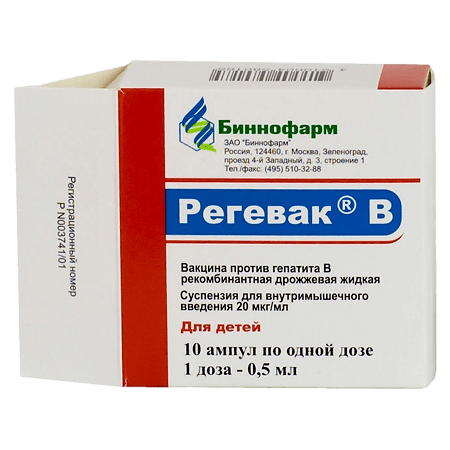
Regevac B for children, 20 µg/ml 0.5 ml 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Hepatitis B prevention:
– in children as part of the national calendar of preventive vaccinations;
– in persons from groups at high risk of infection with hepatitis B virus (children and adults whose families have a carrier of HBsAg or a patient with chronic hepatitis B; Children in orphanages, children’s homes and boarding schools, children and adults who regularly receive blood and blood products, as well as those who are on hemodialysis and oncohematological patients). – In persons who have had contact with material infected with hepatitis B virus;
– In medical workers who have had contact with the blood of patients;
– In persons engaged in the production of immunobiological products from donor and placental blood;
– in students of medical institutions and students of secondary medical schools (primarily graduates);
– in persons who inject drugs.
In addition to the above categories, all other populations should be vaccinated.
Indications
Indications
Prevention of hepatitis B:
— in children within the framework of the national calendar of preventive vaccinations;
– in persons from groups at increased risk of infection with the hepatitis B virus (children and adults in whose families there is a carrier of HBsAg or a patient with chronic hepatitis B; children in orphanages, orphanages and boarding schools; children and adults who regularly receive blood and its preparations, as well as those on hemodialysis and oncohematological patients);
– in persons who have had contact with material infected with the hepatitis B virus;
– for medical workers who have contact with the blood of patients;
— for persons involved in the production of immunobiological preparations from donor and placental blood;
— students of medical institutes and students of secondary medical educational institutions (primarily graduates);
– in people who inject drugs.
In addition to the above categories, all other groups of the population should be vaccinated.
Pharmacological effect
Pharmacological effect
Vaccine for the prevention of hepatitis B.
Special instructions
Special instructions
Considering the possibility of developing immediate allergic reactions in particularly sensitive individuals, vaccinated persons must be provided with medical supervision for 30 minutes after vaccination. Vaccination sites must be provided with anti-shock therapy.
Active ingredient
Active ingredient
Vaccine for the prevention of viral hepatitis B
Composition
Composition
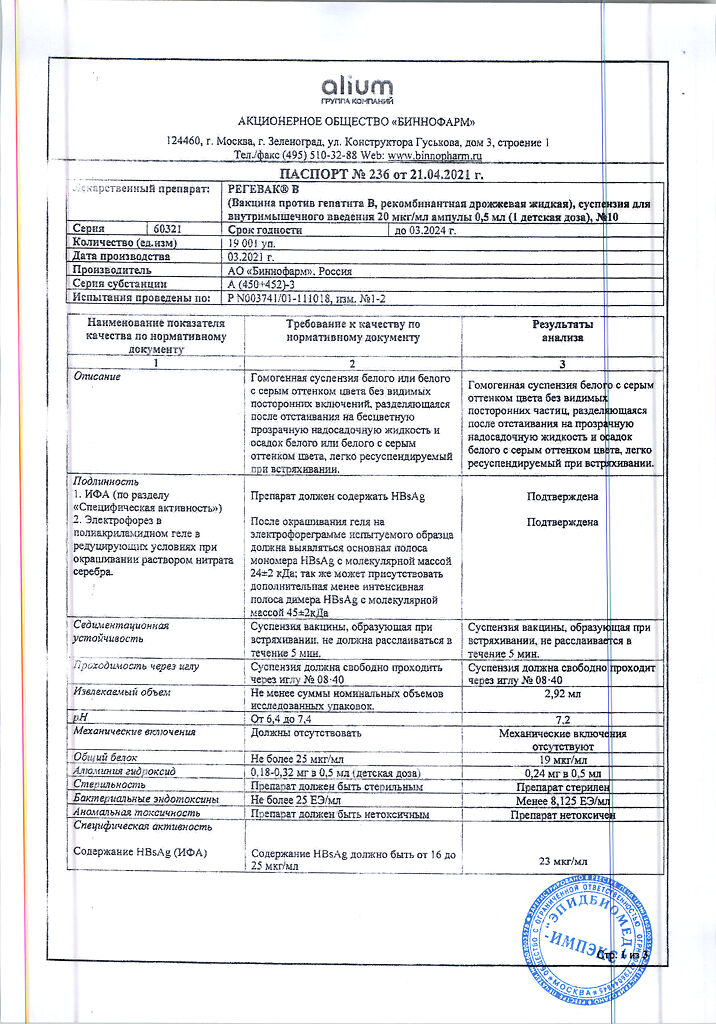
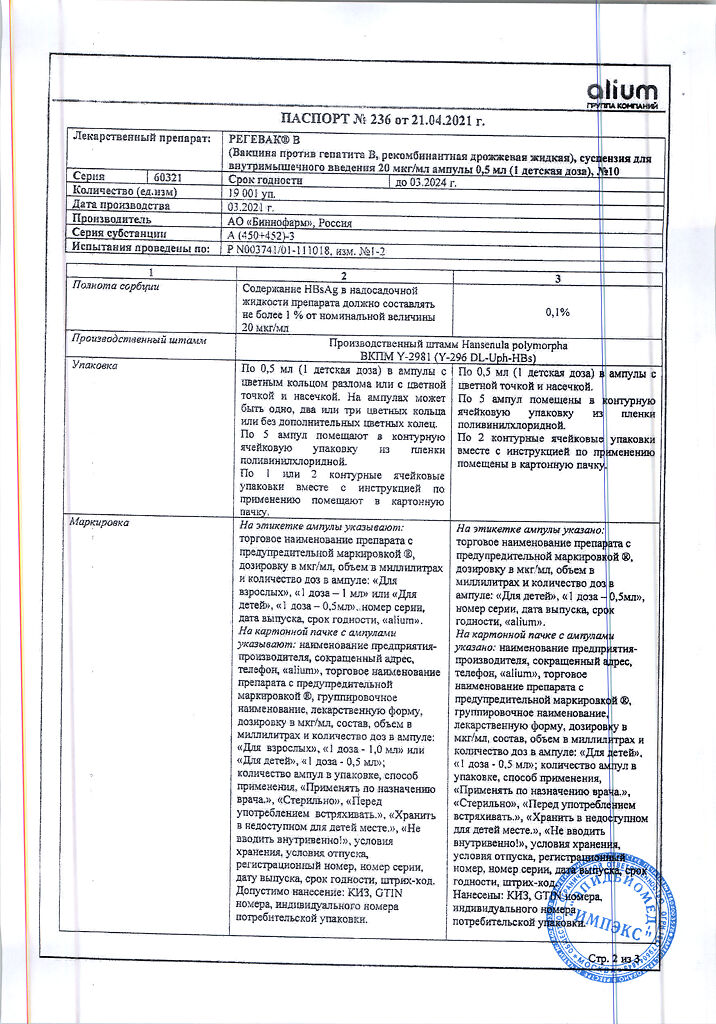
recombinant purified surface antigen of hepatitis B virus (serotype ayw) 10 μg
Excipients:
aluminum hydroxide (sorbent),
thiomersal (preservative) 25 mcg (or does not contain),
buffer components.
Contraindications
Contraindications
– severe reaction (temperature above 40°C, swelling, hyperemia more than 8 mm in diameter at the injection site) or complication to the previous administration of the hepatitis B vaccine;
– acute infectious and non-infectious diseases, chronic diseases in the acute stage – immunization is carried out no earlier than 1 month after recovery (remission);
– hypersensitivity to yeast and other components of the vaccine.
Side Effects
Side Effects
Side effects with the vaccine are rare.
Local reactions: in 5-10% of cases – pain, erythema and induration at the injection site.
Systemic reactions: slight increase in temperature, complaints of malaise, weakness, joint pain, muscle pain, headache, dizziness, nausea, vomiting, abdominal pain.
All reactions to the injection are weak and usually disappear 2-3 days after the injection.
Storage conditions
Storage conditions
The vaccine should be stored and transported in accordance with SP 3.3.2.1248-03 at a temperature of 2°C to 8°C out of the reach of children.
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Binnopharm, Russia
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | The vaccine should be stored and transported in accordance with SP 3.3.2.1248-03 at 2 ° C to 8 ° C out of the reach of children. |
| Manufacturer | Binnopharm, Russia |
| Medication form | suspension |
| Brand | Binnopharm |
Related products
Buy Regevac B for children, 20 µg/ml 0.5 ml 10 pcs with delivery to USA, UK, Europe and over 120 other countries.