No products in the cart.
Ramazid N, tablets 2.5mg+12, 5 mg 30 pcs
€12.88 €10.73
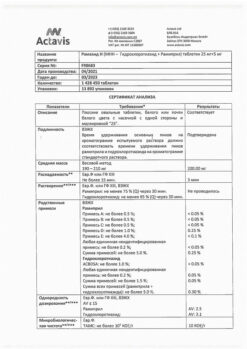
Description
Arterial hypertension (patients who are indicated for combination therapy).
Active ingredient
Active ingredient
Composition
Composition
How to take, the dosage
How to take, the dosage
Ingestion. The dose is adjusted individually. The usual dose for adults is 1 tablet. Ramazid H2.5 mg/12.5 mg per day. If necessary, it can be increased to 1 tablet. Ramazide N 5 mg/25 mg.
In mild to moderate renal dysfunction (CK over 30 mL/min, serum creatinine of approximately 3 mg/dL or 265 µmol/L), the usual dose of the drug is recommended. When creatinine clearance is less than 30 mL/min, the drug is not recommended.
Interaction
Interaction
Ramipril
Aggravates the CNS depressant effect of ethanol. Salt intake with food may reduce the hypotensive effect of ramipril.
Simultaneous use of ramipril and other means that reduce BP (e.g., diuretics, nitrates, tricyclic antidepressants, anesthetics) leads to increased hypotensive effect of ramipril.
The simultaneous use of ramipril and potassium preparations or potassium-saving diuretics, the concentration of lithium in blood serum should be controlled – the risk of toxic effects may cause hyperkalemia.
Vasopressor sympathomimetics (adrenaline, noradrenaline) may reduce the hypotensive effect of ramipril. In this regard, BP level should be carefully monitored during concomitant treatment.
The concomitant administration of ramipril and allopurinol, immunosuppressants, corticosteroids, procainamide, cytostatics increases the possibility of peripheral blood count changes.
The simultaneous administration of ramipril and lithium drugs leads to decreased excretion of l
ACE inhibitors may increase the effect of hypoglycemic agents (e.g., insulin or sulfonylurea derivatives), which in some cases may cause hypoglycemia. In this regard, blood sugar levels should be monitored carefully, especially at the beginning of co-administration.
The simultaneous use of ramipril and nonsteroidal anti-inflammatory drugs (e.g., acetylsalicylic acid and indomethacin) may weaken the hypotensive effect of ramipril. In addition, concomitant use may cause hyperkalemia and increase the risk of renal dysfunction.
Simultaneous use of heparin and ramipril may cause hyperkalemia.
Anaphylactic and anaphylactoid reactions to stinging insect venom (and possibly to other allergens) are more pronounced during treatment with ACE inhibitors.
Hydrochlorothiazide
The simultaneous use of foxglove glycosides with thiazide diuretics increases the possibility of the toxic effects of the glycosides (including increased gastric excitability and hypertension.including increased ventricular excitability) due to the possible development of hypokalemia and hypomagnesemia.
Drugs that intensely bind to proteins (indirect anticoagulants, clofibrate, NSAIDs) increase the diuretic effect of hydrochlorothiazide.
The hypotensive effect of hydrochlorothiazide is enhanced by vasodilators, beta-adrenoblockers, barbiturates, phenothiazines, tricyclic antidepressants, ethanol. Hydrochlorothiazide increases neurotoxicity of salicylates, reduces the effect of oral hypoglycemic drugs, norepinephrine, epinephrine and antipodagric drugs, increases cardiotoxic and neurotoxic effects of lithium preparations, effects of peripheral muscle relaxants, reduces excretion of quinidine.
The simultaneous use of methyldopa may lead to hemolysis. Colestiramine reduces absorption of hydrochlorothiazide.
Hydrochlorothiazide reduces the effect of oral contraceptives.
Special Instructions
Special Instructions
Ramipril
Renal function should be assessed at the start of treatment. Renal function should be carefully monitored during treatment with ramipril especially in patients with impaired renal function, with renal vascular disease (e.g., clinically insignificant renal artery stenosis or hemodynamically significant artery stenosis of the single kidney); heart failure.
The risk of hypersensitivity and allergy-like (anaphylactoid) reactions is increased in patients simultaneously taking ACE inhibitors and undergoing hemodialysis procedures using AN69 dialysis membranes. Similar reactions have been found with low-density lipoprotein apheresis using dextran sulfate, so this method should be avoided during treatment with ACE inhibitors.
During treatment with ramipril, serum urea and creatinine levels may increase in patients with impaired renal function, especially with concomitant treatment with diuretics. In this case, treatment should be continued with lower doses of ramipril or the drug should be discontinued. In patients with impaired renal function the risk of hyperkalemia increases.
In patients with impaired hepatic function due to decreased activity of “hepatic” enzymes the metabolism of ramipril and formation of active metabolite may be delayed. In this regard, treatment of such patients should be started only under close medical supervision.
Caution should be exercised when prescribing ramipril to patients on low-salt or no-salt diet (increased risk of arterial hypotension). Patients with decreased circulating blood volume (as a result of diuretic therapy), dialysis, diarrhea and vomiting may develop symptomatic hypotension.
Transient arterial hypotension is not a contraindication to continue treatment after BP stabilization. If severe arterial hypotension occurs again, the dose should be reduced or the drug should be discontinued.
In patients who have undergone major surgical procedures or who receive other hypotensive agents during general anesthesia, ramipril may cause angiotensin II blockade due to compensatory renin release. If the physician associates the development of arterial hypotension with the above mechanism, the arterial hypotension may be corrected by increasing plasma volume.
In rare cases during treatment with ACE inhibitors agranulocytosis, erythrocytopenia, thrombocytopenia, hemoglobinemia or bone marrow depression are observed. At the beginning and during treatment, it is necessary to monitor the number of white blood cells to detect possible neutropenia/agranulocytosis. More frequent monitoring is recommended in patients with renal insufficiency, with connective tissue diseases (e.g., systemic lupus erythematosus or scleroderma) and in patients simultaneously taking medications that affect hematopoiesis. A count of blood cells should also be performed if there are clinical signs of neutropenia/agranulocytosis and increased bleeding.
In patients with arterial hypertension during treatment with ramipril an increase in serum potassium levels is rarely observed. The risk of hyperkalemia increases with chronic heart failure, concomitant treatment with potassium-saving diuretics (spironolactone, amiloride, triamterene) and administration of potassium preparations.
When ACE inhibitors are used during desensitization therapy to wasp or bee venom, anaphylactoid reactions (e.g., arterial hypotension, dyspnea, vomiting, skin rash) may occur, which may be life-threatening. Hypersensitivity reactions can occur with insect stings (e.g. bee or wasp stings). If desensitizing treatment with bee or wasp venom is necessary, ACE inhibitors should be stopped and treatment should be continued with suitable drugs from other groups.
When treating with Ramazide N it is necessary to be careful when driving motor transport and engaging in other potentially dangerous activities requiring increased concentration and rapid psychomotor reactions (dizziness is possible, especially after the initial dose of ACE inhibitor in patients taking diuretic drugs). Patients are advised to refrain from driving and operating machinery until the response to treatment is clear.
Hydrochlorthiazide
Kalium-saving diuretics, K+ and Mg2+ salts are prescribed to prevent K+ and Mg2+ deficiency. Regular monitoring of plasma potassium, glucose, uric acid, lipids, and creatinine is necessary.
Contraindications
Contraindications
Ramipril
– hypersensitivity to ramipril and any other ingredient of the drug or other ACE inhibitors;
– history of angioedema, including that associated with prior therapy with ACE inhibitors;
/p>
– hemodynamically significant bilateral renal artery stenosis;
– arterial stenosis of the sole kidney;
– post renal transplant condition;
– hemodialysis;
– renal failure (CK less than 20 ml/min.);
– hemodynamically significant aortic or mitral stenosis (risk of excessive BP decrease with subsequent impairment of renal function);
– hypertrophic obstructive cardiomyopathy;
– primary hyperaldosteronism;
– pregnancy and lactation;
– age less than 18 years (efficacy and safety not established).
With caution: severe coronary and cerebral artery lesions (risk of reduced blood flow with excessive BP reduction), unstable angina pectoris, severe ventricular arrhythmias, chronic heart failure stage IV, decompensated “pulmonary heart”, renal and/or liver failure, hyperkalemia, hyponatremia (including against diuretics and salt restricted diet), conditions accompanied by decreased circulating blood volume (incl.including diarrhea, vomiting), systemic connective tissue diseases, diabetes, suppression of cerebrospinal circulation, advanced age.
Hydrochlorothiazide
– hypersensitivity to the drug;
– gout;
– diabetes mellitus (severe forms);
– chronic renal failure (creatinine clearance less than 20-30 ml/min, anuria);
– refractory hypokalemia;
– hypercalcemia;
– hyponatremia;
– pregnancy (1st trimester);
– lactation.
With caution: hypokalemia, hyponatremia, hypercalcemia, coronary heart disease, cirrhosis of the liver, advanced age.
Side effects
Side effects
Ramipril
Cardiovascular system disorders: decreased BP, orthostatic hypotension, orthostatic collapse, tachycardia, rarely – arrhythmia, angina pectoris, myocardial infarction. Urinary system disorders: development or worsening of symptoms of renal failure, proteinuria, decreased volume of urine, decreased libido.
CNS disorders: cerebral ischemia, stroke, dizziness, headache, weakness, somnolence, paresthesia, nervous irritability, anxiety, tremor, muscle spasm, mood disorders, in high doses – insomnia, anxiety, depression, confusion, fainting.
Sensory organs: vestibular disorders, disorders of taste (e.g., metallic taste), smell, hearing and vision, tinnitus.
Digestive system disorders: nausea, vomiting, diarrhea or constipation, epigastric pain, intestinal obstruction, pancreatitis, hepatitis, cholestatic jaundice, liver function disorders with development of liver failure, dry mouth, thirst, decreased appetite, stomatitis, glossitis.
Respiratory system: “dry” cough, bronchospasm, shortness of breath, rhinorrhea, rhinitis, sinusitis, bronchitis.
Allergic reactions: skin rash, pruritus, urticaria, conjunctivitis, photosensitization; angioedema of the face, extremities, lips, tongue, pharynx and/or larynx, exfoliative dermatitis, erythema multiforme exudative erythema (including Stevens-Joint syndrome).Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell’s syndrome), vesicles, serositis, onycholysis, vasculitis, myositis, myalgia, arthralgia, arthritis, eosinophilia.
Others: seizures, alopecia, hyperthermia, increased sweating.
Laboratory measures: hypercreatininemia, increased urea nitrogen level, increased “liver” transaminase activity, hyperbilirubinemia, hyperkalemia, hyponatremia, appearance of antinuclear antibodies.
Fetal effects: impaired fetal function, decreased fetal and neonatal BP, impaired renal function, hyperkalemia, skull bone hypoplasia, oligohydramnion, limb contracture, skull bone deformity, lung hypoplasia.
Hydrochlorthiazide
Water-electrolyte and acid-base balance: possible development of hypokalemia and hypochloremic alkalosis (dry mouth, increased thirst, heart rhythm disturbances, mood and mental changes, muscle cramps or pain, nausea, vomiting, weakness; hypochloremic alkalosis may lead to hepatic encephalopathy or hepatic coma), hyponatremia (confusion, convulsions, apathy, slowed thinking process, fatigue, irritability), hypomagnesemia (arrhythmia).
Hematopoietic system disorders: agranulocytosis, thrombocytopenia, hemolytic and aplastic anemia, leukocytopenia.
Cardiovascular system: arrhythmia, orthostatic hypotension, tachycardia.
Digestive system disorders: cholecystitis, pancreatitis, jaundice, diarrhea, sialadenitis, constipation, anorexia, epigastric pain.
Metabolism disorders: hyperglycemia, glucosuria, hyperuricemia, aggravation of gout.
Allergic reactions: skin rash, purpura, necrotizing vasculitis, Stevens-Johnson syndrome, respiratory distress (pneumonitis, noncardiac pulmonary edema), photosensitization; anaphylactic reactions (up to and including life-threatening anaphylactic shock).
Overdose
Overdose
Symptoms: marked BP decrease, bradycardia, shock, water-electrolyte imbalance, acute renal failure, stupor, dry mouth, weakness, somnolence.
Treatment: the patient should be in horizontal position with elevated legs, in mild cases of overdose – gastric lavage, administration of adsorbents and sodium sulfate (preferably within the first 30 minutes after taking the drug). If BP decreases – intravenous administration of catecholamines, angiotensin II; if bradycardia – use pacing agent. The drug is not excreted during hemodialysis.
Pregnancy use
Pregnancy use
Similarities
Similarities
Additional information
| Shelf life | 3 years. The drug should not be used after the expiration date stated on the package. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25°C. Keep out of reach of children! |
| Manufacturer | Actavis JSC, Malta |
| Medication form | pills |
| Brand | Actavis JSC |
Other forms…
Related products
Buy Ramazid N, tablets 2.5mg+12, 5 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.